Photovoltaics

Perovskites, which are generically defined by an ABX3 stoichiometry and a corner-shared anion octahedral geometry, are ubiquitous in modern devices because their wide compositional range and structural variability gives rise to useful applications as piezoelectrics, pyroelectrics, ferroelectrics, superconductors, colossal magnetoresistors, proton conductors, catalysts, spin-dependent transport media, and photovoltaics. Indeed, the optical bandgaps, high absorption coefficients, long electron–hole diffusion lengths, and large dielectric constants make halide perovskites particularly interesting for photovoltaic devices.
One of the most promising contenders in the race for efficient, cost-effective solar materials is the perovskite solar cell. The high coefficient of optical absorption and large dielectric constant of many perovskites make them highly useful as energy conversion materials. Due to their light-absorbing properties, they have become a hot topic in solar energy and sustainability. Perovskite solar cells have skyrocketed in efficiency compared to all other older, competing devices. The rapid advances in this field make them remarkable candidates for applied research.
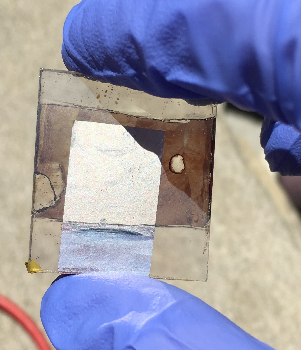
The solar-cell devices in this work consist of an etched glass substrate coated with indium-tin oxide, a nickel oxide layer, a hybrid organic-inorganic perovskite layer, a zinc oxide layer, and an aluminum electrode layer. The (CH3NH3)PbI3 perovskite is the optically active layer, akin to the electron-donor material in heterojunction solar cells, absorbing light and injecting electrons (and holes) into conducting media. The addition of Al to the n-type ZnO layer can increase both conductivity and optical transmission; therefore, this research seeks to produce functional lead halide perovskite solar cells and observe the changes in electrical conductivity and energy conversion efficiency when the zinc oxide layer is doped with aluminum. All layers were fabricated using solution processing methods.
The greatest challenge was preventing the solar cells from short-circuiting due to contact between the Al electrode and the ITO coating. With structural modifications via etching and changing the area of each layer, the solar cells are now able to collect voltage in the sunlight.
It was found that pinholes formed in the perovskite films due to surface contaminants and evaporation upon annealing. These pinholes allowed electrons and holes to recombine, ceasing their flow. Forming the perovskite layer in a two-step process, in which lead iodide and methylammonium iodide precursors were deposited in separate steps, largely solved this problem, yielding increased coverage and higher energy-conversion efficiencies. Using this method in a glove box environment produced our most efficient solar cells. The highest performing device had an open-circuit voltage of 200 mV and an efficiency of 13%.