Article by Research Scholar Olya Mass and Research Program Manager John Hall, qDNA Research Group
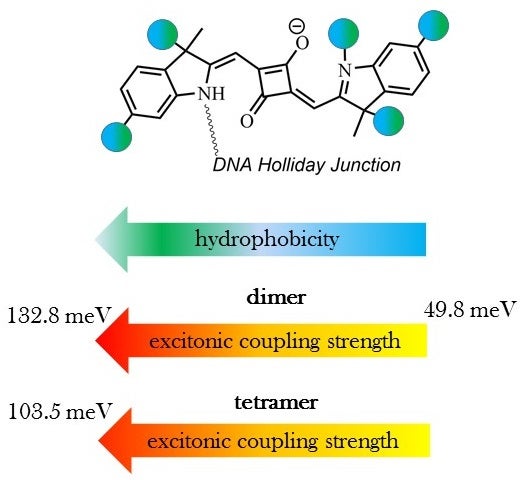
Dye hydrophobicity–literally a tedency to repel from water–influences the strength of excitonic coupling in squaraine dye aggregates. By substituting different functional groups on these dyes, the degree of hydrophobicity, and therefore coupling strength, can be fine-tuned.
What did the Scientists/ Engineers Discover?
Molecular dye aggregates provide a promising material system for the development of technologies such as light harvesting, optoelectronics, and quantum computing. Their usage relies in part on controlling the strength of their coupling when aggregated. For dyes in close proximity coupling occurs through the sharing of an exciton; in other words, sharing the excited state of a dye that has absorbed a photon of light. The strength of excitonic coupling is inversely proportional to the distance between the dyes. The Quantum DNA (qDNA) Research Group has shown previously that covalently bonding dyes to a DNA scaffold provides a means to control dye spacing on a subnanometer scale. Here, Micron School of Materials Science and Engineering (MSMSE) Research Scholar Olya Mass and colleagues investigated the influence of dye hydrophobicity on the strength of excitonic coupling in DNA-templated squaraine dye aggregates.
Squaraine dyes are a class of organic dyes that exhibit interesting spectral properties, stability, and diversity of chemical modifications. Adding different functional groups–i.e., an atom or group of atoms with distinct chemical properties–to a dye is a means of chemical modification that enables assessing resultant changes in the photophysical, as well as chemical properties of the dye. In the present study, six squaraines of differing hydrophobicity (as determined by the different functional groups attached and their placement) were assembled in different aggregate configurations and their key properties were examined. Spectral data and modeling using an in-house simulation tool showed that the strength of excitonic coupling strongly correlated with a dye’s hydrophobic surface area as determined by the type of functional group added and its location(s) on the dye.
Impact
The research findings of Mass and colleagues provide a deeper insight into how dye chemical and physical structures influence excitonic coupling in dye aggregates that use DNA as a scaffold. In addition, the findings contribute to our understanding of design rules for exciton-based materials and devices.
Study Investigators
- Olga A. Mass, MSMSE, Boise State University
- Christopher K. Wilson, MSMSE, Boise State University
- German Barcenas, MSMSE, Boise State University
- Ewald A. Terpetschnig, SETA BioMedicals, LLC
- Olena M. Obukhova, State Scientific Institution “Institute for Single Crystals” of National Academy of Sciences of Ukraine
- Olga S. Kolosova, State Scientific Institution “Institute for
Single Crystals” of National Academy of Sciences of Ukraine - Anatoliy L. Tatarets, State Scientific Institution “Institute for
Single Crystals” of National Academy of Sciences of Ukraine - Lan Li, MSMSE, Boise State University
- Bernard Yurke, MSMSE and Department of Electrical and Computer Engineering (ECE), Boise State University
- William B. Knowlton, MSMSE and ECE, Boise State University
- Ryan D. Pensack, MSMSE, Boise State University
- Jeunghoon Lee, MSMSE and Department of Chemistry and Biochemistry, Boise State University
Publication Citation
Olga A. Mass, Christopher K. Wilson, German Barcenas, Ewald A. Terpetschnig, Olena M. Obukhova, Olga S. Kolosova, Anatoliy L. Tatarets, Lan Li, Bernard Yurke, William B. Knowlton, Ryan D. Pensack, and Jeunghoon Lee, The Influence of Hydrophobicity on Excitonic Coupling in DNA-Templated Indolenine Squaraine Dye Aggregates, Journal of Physical Chemistry C, 126, 3475-3488 (2022). doi.org/10.1021/acs.jpcc.1c08981.
Funding
| Funding Agency | Grant/Contract Number | Role of Funding |
|---|---|---|
| U.S. Department of Energy (DOE), Office of Basic Energy Sciences, Materials Sciences and Engineering Division and DOE’s Established Program to Stimulate Competitive Research (EPSCoR) | DOE award DE-SC0020089 | Primary funding for Mass et al. 2021 |
| National Institutes of Health (NIH), MJ Murdock Charitable Trust, and Idaho State Board of Education (to the Biomolecular Research Center at Boise State) | NIH award Nos. P20GM103408 and P20GM109095 | Use of the circular dichroism spectrometer |
Disclaimer
This research was supported wholly by the U.S. Department of Energy (DOE), Office of Basic Energy Sciences, Materials Sciences and Engineering Division, and DOE’s Established Program to Stimulate Competitive Research (EPSCoR) (Award DE-SC0020089), except for the use of the circular dichroism spectrometer that was funded in part by the National Institutes of Health (NIH). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the DOE and NIH.