Madison Sullivan, Rachael Neckels, Mariah Provost, Spencer Goering, Dr. Jim Browning, Dr. Ken Cornell
Background
Chronic wounds affect approximately six million people in the United States, and are most common in elderly and immunocomrpomised patients. Opportunistic pathogenic bacteria often exist in these wounds in the form of biofilms composed of layers of extracellular polymeric substances (EPS). These biofilms can lead to chronic wounds that fail to heal because the defined stages of healing are inhibited and inflammation persists. This requires the wound to be cleaned and debrided in order to try and sponsor normal healing. Current methods of debridement for chronic wounds are time consuming, painful, and expensive. Cold atmospheric-pressure plasmas (CAP) have shown some promise in microbial decontamination of wounds. Here we report on our studies using a novel CAP array device to inactivate pathogens grown in porcine explants that model the processes found in chronic wounds since they resemble human tissue.
Hypothesis
CAP treatment will kill wound biofilm bacteria and be an effective alternative for chronic wound debridement to speed up the healing process.
Methods
- Innoculate explant with 1μL of bacteria
- Establish biofilm in the model wound
- CAP treatment
- Disaggregate tissue, plate out for CFU
- Analyze data for % total CFU vs. Treatment time
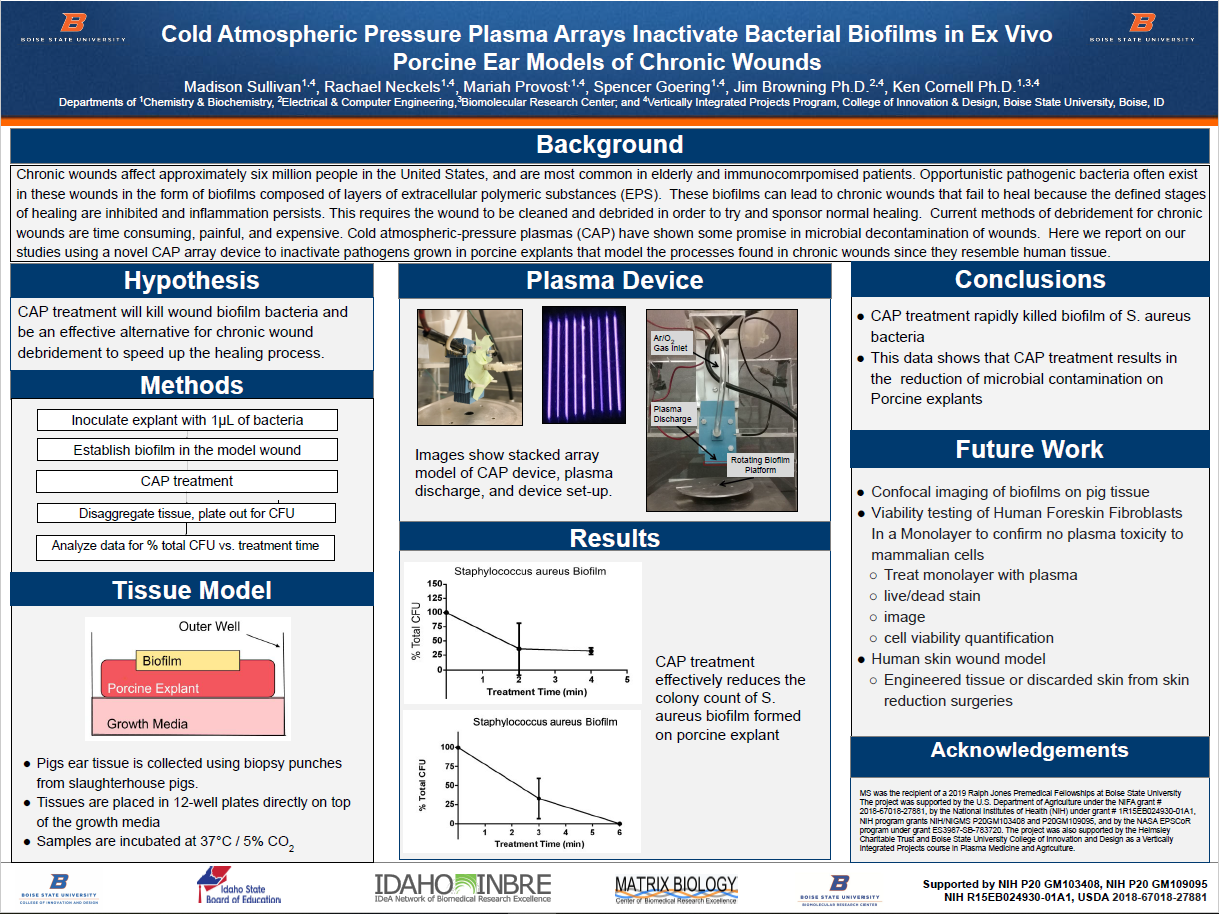
Tissue Model
- Pigs ear tissue is collected using biopsy punches from slaughterhouse pigs.
- Tissues are placed in 12-well plates directly on top of the growth media
- Samples are incubated at 37°C / 5% CO2
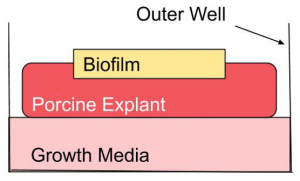
Plasma Device
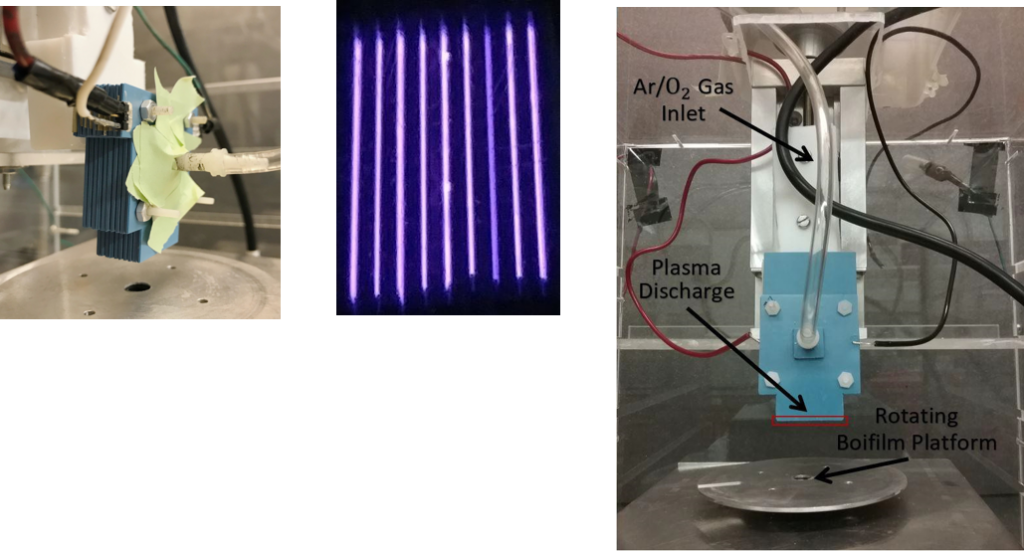
Results
Conclusions
- CAP treatment rapidly killed biofilm of S. aureus bacteria
- This data shows that CAP treatment results in the reduction of microbial contamination on Porcine explants
Future Work
- Confocal imaging of biofilms on pig tissue
- Viability testing of Human Foreskin Fibroblasts In a Monolayer to confirm no plasma toxicity to mammalian cells
- Treat monolayer with plasma
- live/dead stain
- image
- cell viability quantification
- Human skin wound model
- Engineered tissue or discarded skin from skin reduction surgeries
Acknowledgements
MS was the recipient of a 2019 Ralph Jones Premedical Fellowships at Boise State University The project was supported by the U.S. Department of Agriculture under the NIFA grant #2018-67018-27881, by the National Institutes of Health (NIH) under grant # 1R15EB024930-01A1, NIH program grants NIH/NIGMS P20GM103408 and P20GM109095, and by the NASA EPSCoR program under grant ES3987-SB-783720. The project was also supported by the Helmsley Charitable Trust and Boise State University College of Innovation and Design as a Vertically Integrated Projects course in Plasma Medicine and Agriculture.
Additional Information
For questions or comments about this research, contact Madison Sullivan at madisonsullivan@u.boisestate.edu.