Rebecca K. Torres, Maranda Cantrell, Dr. Owen M. McDougal

Abstract
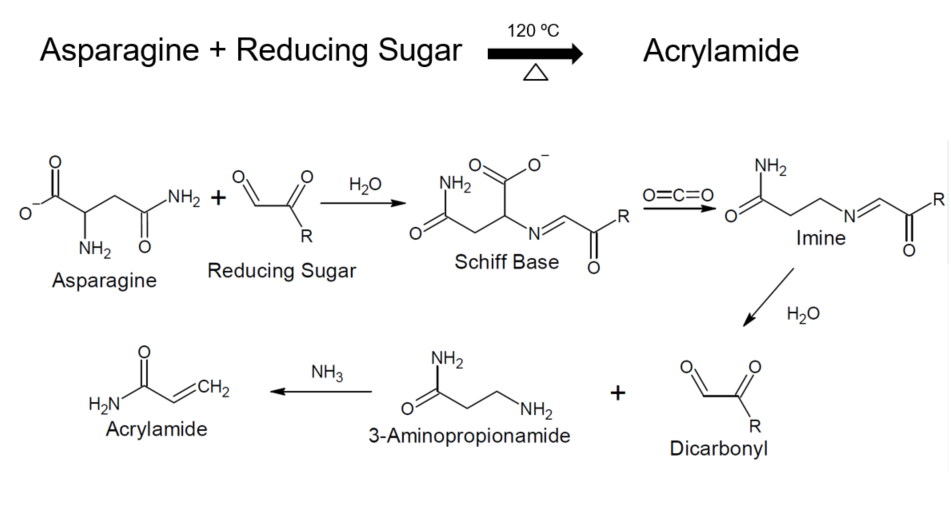
Acrylamide is a suspected carcinogen required to be listed on food labels in California in accordance with Proposition 65. Certain foods that are cooked at elevated levels convert amino acids, such as arginine, asparagine, and lysine, into acrylamide by way of the Maillard reaction. Potato chips, French fries, breakfast cereals, coffee, and many other common foods contain acrylamide, although no definitive impact on human health has yet to be established. To inform the public of acrylamide levels in food, an efficient method for detection is required. We propose to use near Infrared (NIR) spectroscopy as an easily implement testing method for food acrylamide levels. To validate the NIR results, Liquid Chromatography-Mass Spectrometry (LC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS) will be used for qualitative and quantitative analysis of acrylamide levels in light, medium, and dark roast coffee.
Introduction

Background
- 1968: California Proposition 65
- 1986 & 1995: Acrylamide suspected carcinogen in rats
- 2011: Proposition 65 – acrylamide added to the list
- 2018: Acrylamide added to food labels
- 2019: California judge rules cancer warning for coffee
- Idaho = potato state; export product acrylamide testing
- Current markets requiring acrylamide testing = California & EU
- Testing methods for acrylamide = HPLC & GC-MS.
- Testing methods are complex, expensive, and time consuming.
- Near Infrared spectroscopy is simple, rapid and can be performed with minimal sample preparation and employee training.
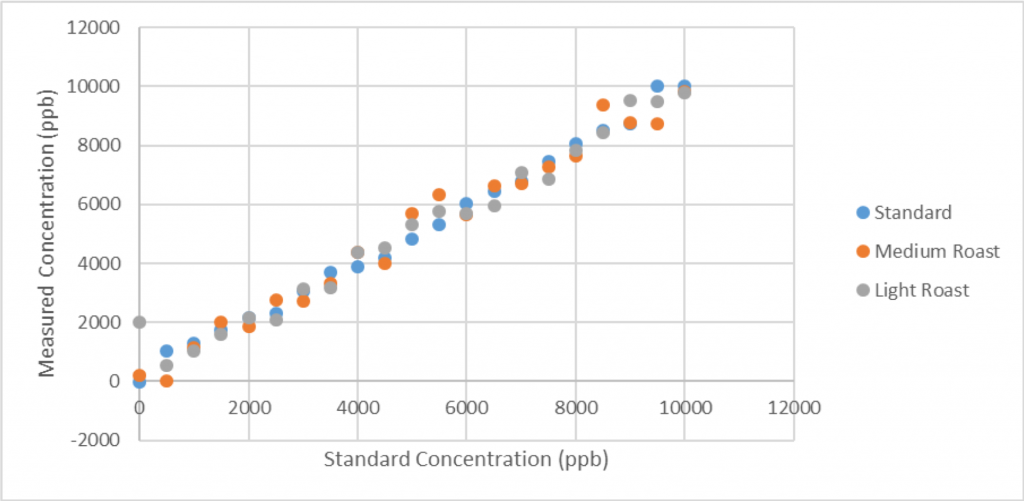
- Hypothesis: Darker roast = more acrylamide
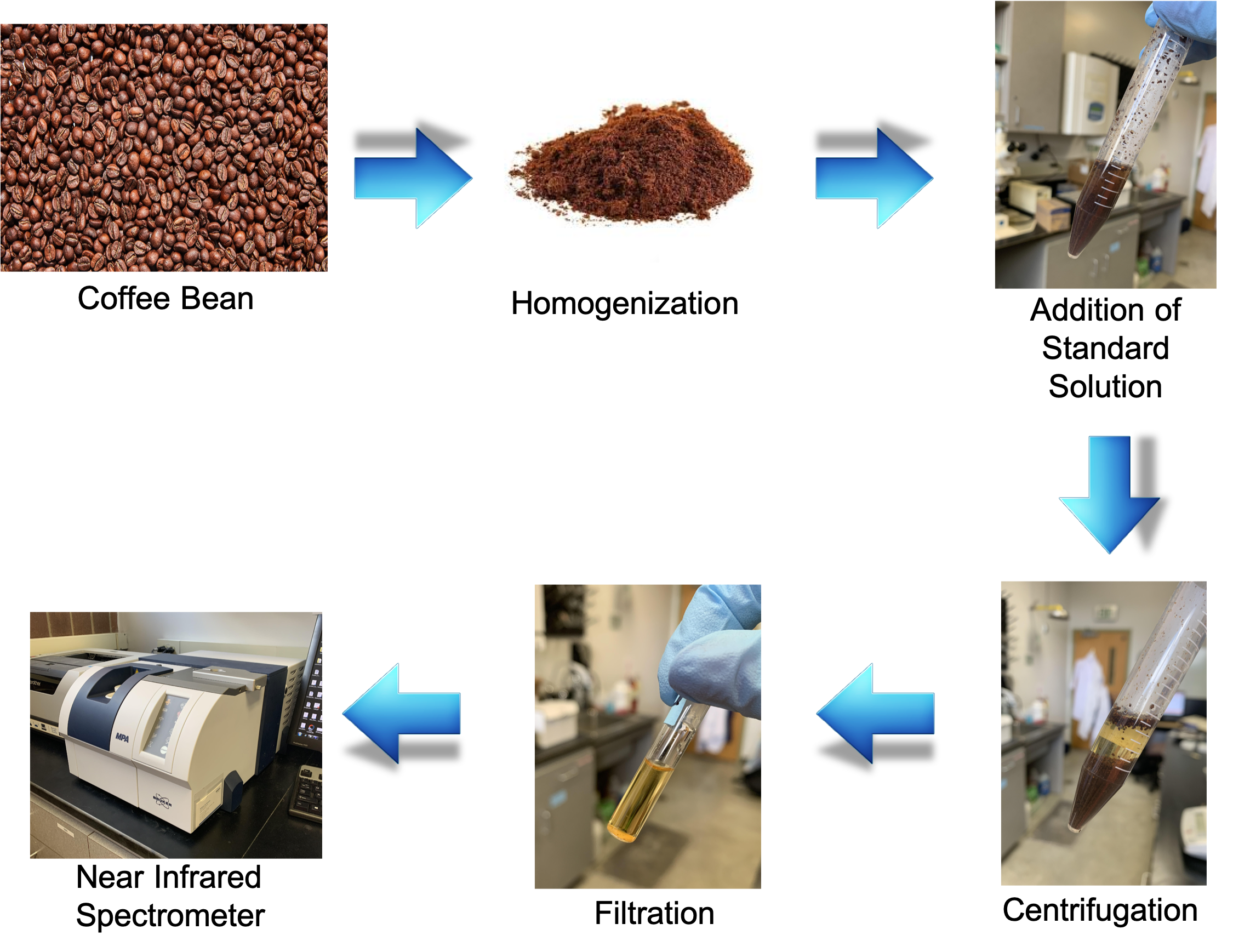
Methods
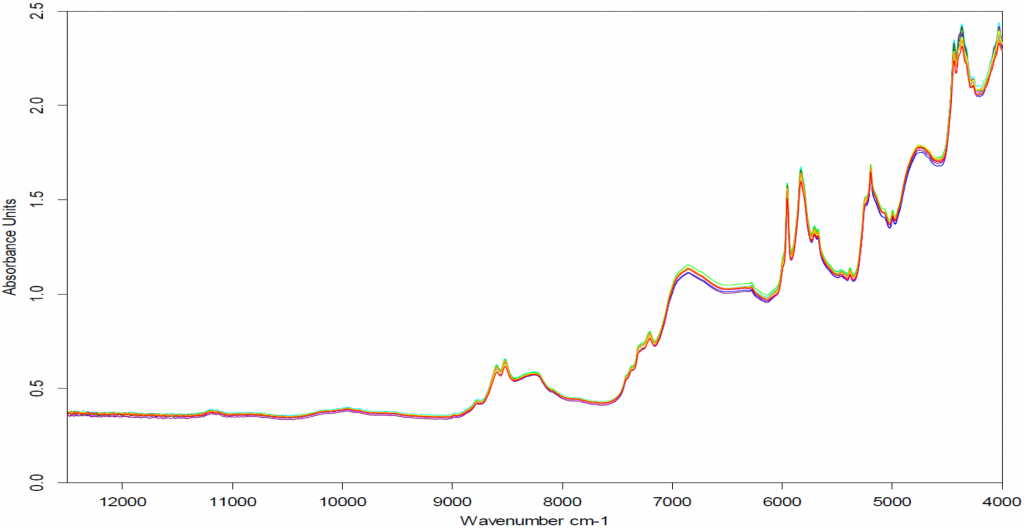
Twenty samples of homogenized coffee grounds were spiked with standard solutions with known concentrations of 50, 1000, 1500, 2000, 2500, 3000, 3500, 4000, 4500, 5000, 5500, 6000, 6500, 7000, 7500, 8000, 8500, 9000, 9500, and 10,000 parts per billion (ppb) acrylamide in acetonitrile.
Mallard Reaction
Results
Future Directions
Validation of Near Infrared spectroscopy results using LC-MS and GC-MS methods will be helpful for the qualitative and quantitative analysis of acrylamide levels in light, medium, and dark roast coffee. Extrapolate method to evaluation of acrylamide content in potato products.
Acknowledgements
The project described was funded by the Institute for STEM & Diversity Initiatives at Boise State University, and the Idaho State Department of Agriculture Specialty Crop Block Grant Program.
References
- https://oehha.ca.gov/proposition-65/general-info/acrylamide accessed 1/26/20
- Adedipe, O. E., et al (2017). MCJ 1851-1854
- Razia, S., et al (2016). IFRJ 23(5), 2188-2189
- Surma, M., et al (2016). JAFC 131, 99-100
- Mastovska, K., et al (2006). JAFC
Additional Information
For questions or comments about this research, contact Becca Torres at rebeccatorres@u.boisestate.edu.