Julie Wagner, Caleb Leach, Tyler Smith, Colton Brodock, Dr. Ken Cornell
Abstract
Giardia intestinalis is a parasitic protozoan that is one of the most common causes of intestinal illness, with hundreds of millions of cases every year. Increasingly, drug resistant strains of Giardia intestinalis have been discovered. Thus, there is an urgent need to develop novel anti-parasitic therapies. A potential target for new drug development is the parasite specific enzyme 5’ methylthioadenosine nucleosidase (MTN). MTN plays a central role in the methionine/purine salvage pathways required for G. intestinalis survival. Initial in silico computational studies identified over 60 potential MTN inhibitors from several libraries of compounds containing hundreds of thousands of inhibitors. Some of these compounds are already FDA approved and currently on the market. For this project, we purified recombinant Giardia MTN and analyzed select sets of potential MTN inhibitors using a UV spectrophotometric assay. Percent inhibition was used to determine how effective the inhibitor is against MTN. Additionally, we conducted Ki studies to determine if the inhibitor is acting allosterically, competitively, or mixed. The goal of the Ki study is to determine if the inhibitor functions at a concentration similar to current anti-parasitic on the market to warrant further testing.
Background
Symptoms
Symptoms of Giardia typically after 1 to 3 weeks of ingestion1,3
- Diarrhea
- Discomfort in abdominal region
- Nausea or vomiting
- Dehydration
Transmission of Giardia1,3
- Most commonly by ingestion of contaminated water
- Ingesting Giardia from objects that have encountered feces
- Eating uncooked food that contains Giardia
- Personal contact with someone who has G. intestinalis
Morbidity4
- An estimated 1.2 million cases of giardiasis occur in the U.S. annually
- Rates of giardiasis are highest in children (ages 1-4)
- In Idaho, incidence rate is 7.6-10.0 per capita
- Northwestern states have highest rates of Giardia cases per capita
Hypothesis
Inhibitors of MTN that were identified through in silico analysis will be useful anti-parasitic drugs.
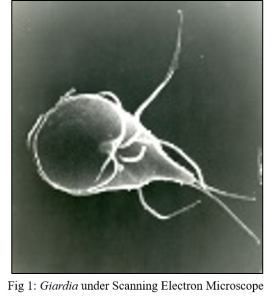
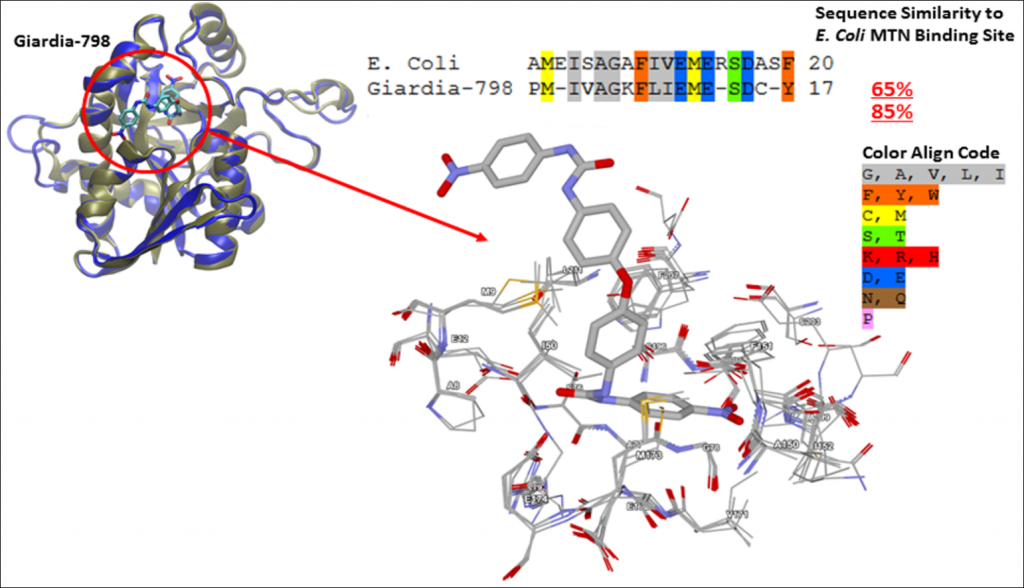
Enzyme GI 798 and SAM Pathway
UV Spectrophotometer Assay
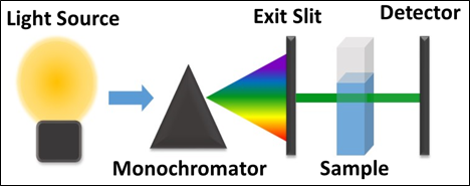
- UV Assay was used to determine how effective the inhibitor was against GI 798 MTN by enzyme activity
- Measured the change in absorbance at 275 nm
- This indicates that MTA was cleaved by MTN
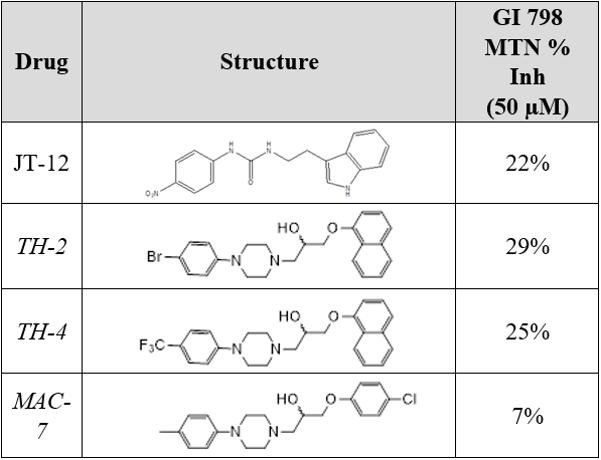
Percent Inhibition Results
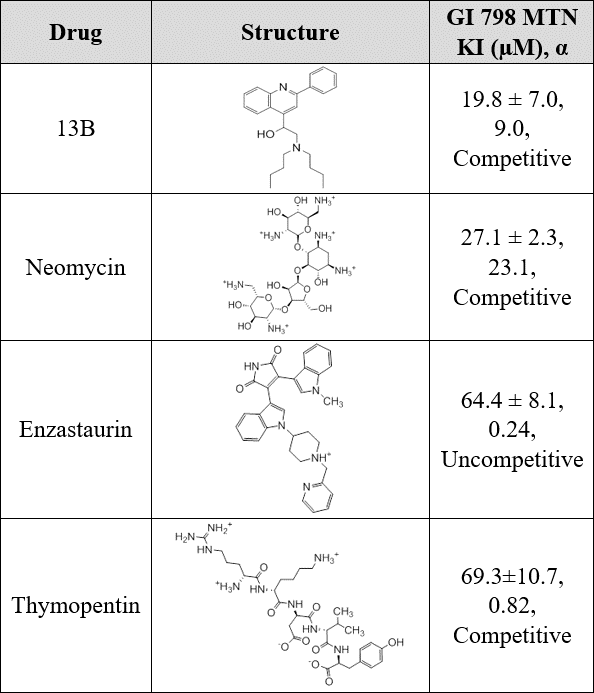
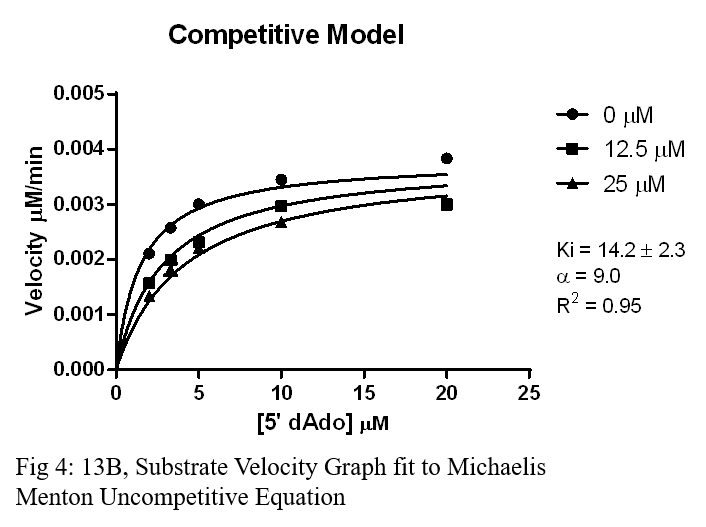
Ki Results
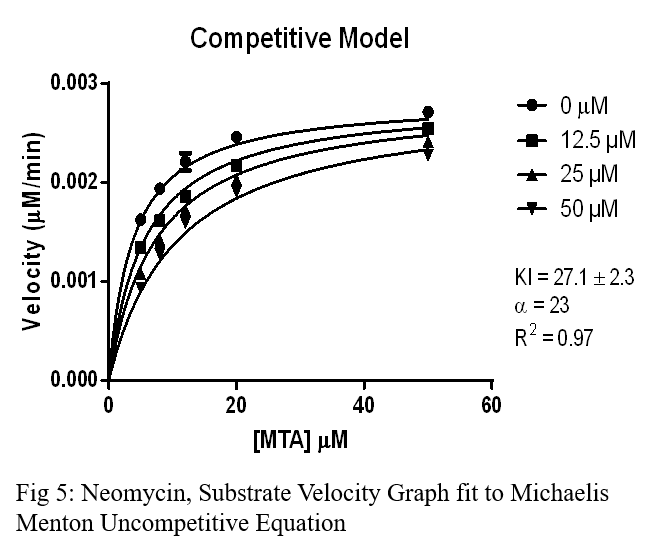
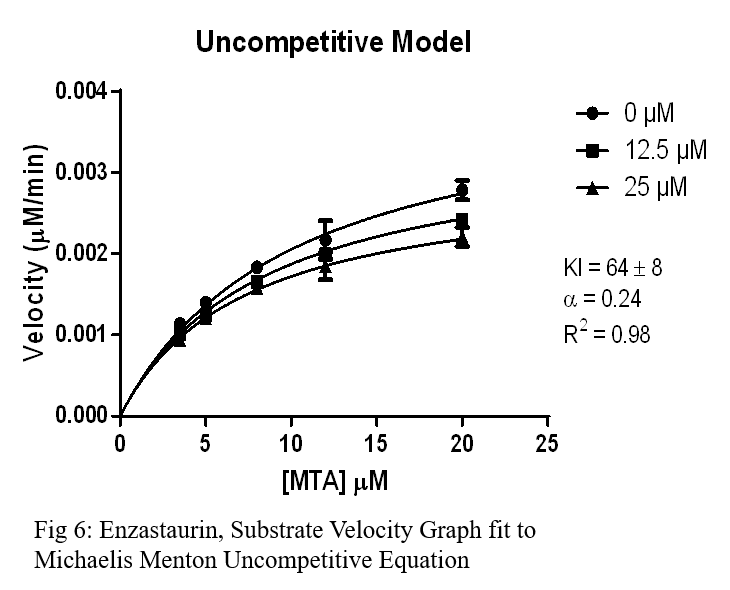
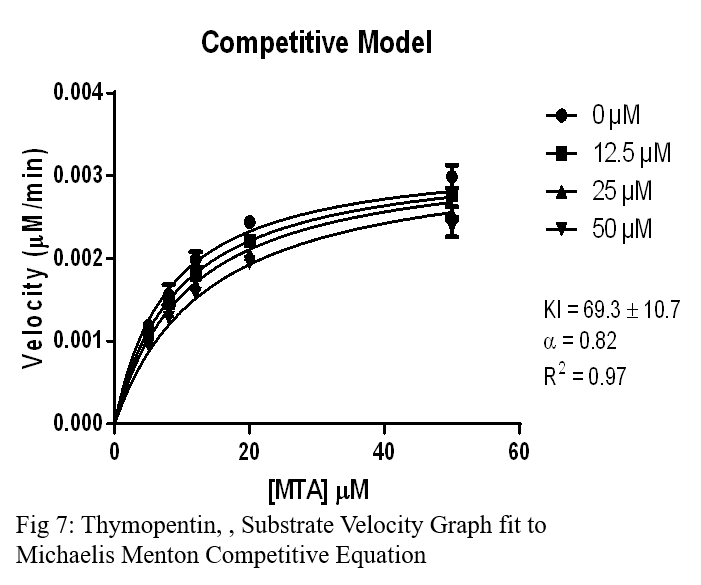
Conclusion
- JT-12, TH-2, and TH-4 all showed promising percent inhibitions, they will all be further analyzed via Ki
- Consequently, MAC-7, with a lower percent inhibition will not be further studied
- 13B, a competitive drug, showed the most promising results with the lowest Ki out of the four, will be further analyzed
- Neomycin also showed competitive inhibition, but had a higher Ki
- Thymopentin was the third competitive molecule, but had a much higher Ki
- Enzastaurin had a similarly high Ki, but showed uncompetitive inhibition which can yield promising results
Future Work
- Complete percent inhibition on remaining drug series
- Ki studies will be completed on drugs showing great than ten percent inhibition
- Test inhibitors against MTAP and mammalian cell lines
- Move all promising inhibitors to clinical trials
Funding
JW received a research fellowship from the Bridges to Baccalaureate program supported by the National Institute of General Medical Sciences of the National Institutes of Health grant R25GM123927. TS is a prior recipient of INBRE and Ralph Jones summer research fellowships at Boise State University. The project was supported by NIH grant 1R15GM125065-01, and NIH/NIGMS program grants P20GM103408 and P20GM109095. The project was also supported by the Biomolecular Center at Boise State University, and the Institute for Translational Health Sciences (ITHS).
References
- Centers for Disease Control and Prevention. (2015, July 21). Illness. Retrieved April 5, 2020, from Centers for Disease Control and Prevention: https://www.cdc.gov/parasites/giardia/illness.html
- Centers for Disease Control and Prevention. (2015, July 21). Treatment. Retrieved April 5, 2020, from Centers for Disease Control and Prevention: https://www.cdc.gov/parasites/giardia/treatment.html
- Gardener, T. B., & Hill, D. R. (2001, January). Treatment of Giardiasis. Clinical Microbiology Reviews, 14(1), 114-128.
- Painter, J. E., Gargano, J. W., Collier S. A., Yoder J. S. (2015, May 1). Giardiasis Surveillance — United States, 2011–2012. MMWR Surveillance Summaries, 64(3), 15-25.
Additional Information
For questions or comments about this research, contact Julie Wagner at juliewagner@u.boisestate.edu.