Leyla Cufurovic, Adriana Rodriguez, Omid Mohammad Mousa, Dr. Juliette Tinker

Abstract
According to the CDC, Salmonella is responsible for about 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States every year. Food is the source for most of these illnesses. Salmonella enterica is common in cattle and has a number of serovars that are pathogenic in humans. Many Salmonella serovars encode for the AB5 toxin, or ArtAB, which is similar in structure to pertussis toxin. The pentameric B subunit is non-covalently linked to the A subunit, thus allowing separation of the receptor-binding B subunit from the holotoxin. We hypothesize that being able to extract and use ArtAB or the non-toxic ArtB subunit for vaccines could decrease prevalence of Salmonella infections in cows and potentially prevent transmission from cattle and agriculture to humans. The B subunit can be transformed into E. coli using a vector plasmid and then purified and extracted using D-galactose and fetuin affinity chromatography methods. Our results show that we have successfully cloned the B subunit into a plasmid, transformed the plasmid into E. coli, and had ArtB protein expression. Further research will examine the toxicity of ArtAB and ArtB to determine whether they are suitable for use in vaccines.
Background
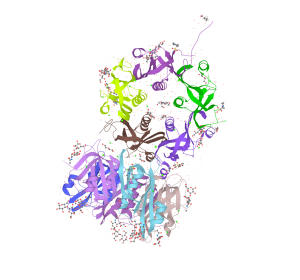
Salmonella enterica Typhimurium is a serious human pathogen that significantly contributes to foodborne illnesses such as salmonellosis, especially in respect to produce and meat. Its pathogenicity can be explained by several virulence factors, potentially including a novel AB5-type toxin found in certain strains.
ArtAB, which is an AB5-type toxin, is a protein expressed by salmonella enterica Typhimurium strain DT104 that can disrupt host signal transduction pathways. This strain has shown multi-drug resistance and hyper-virulence attributed to the ArtAB toxin. Despite its high pathogenicity, DT104 does not show enhanced methods of immune evasion compared to other strains and therefore the mechanisms it uses to quickly spread and infect both bovine and human hosts is still unknown.
Further studying S. Typhimurium strain DT104 in order to understand its mechanistic pathways of pathogenicity is significant in the prevention of food-borne illnesses. Specifically, by studying ArtAB toxin, we can potentially derive a vaccine to be used in both bovine and human recipients.
Methods and Results

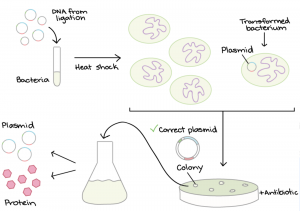

Conclusions and Future Plans
Conclusions
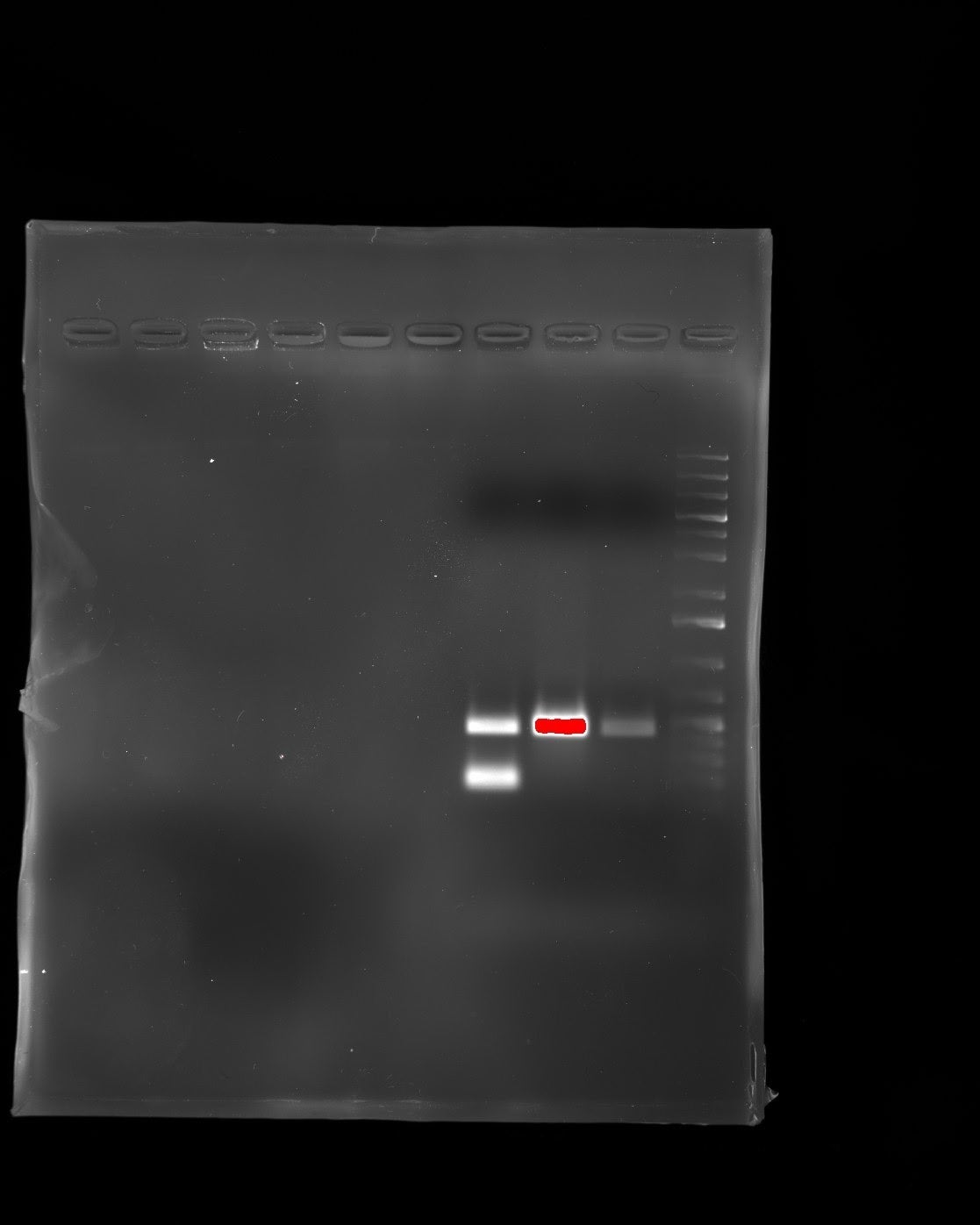
- We have successfully cloned artB and purified ArtB from Salmonella enterica Typhimurium DT104 using D-galactose affinity chromatography.
- The plasmid has shown to be producing large amounts of ArtB
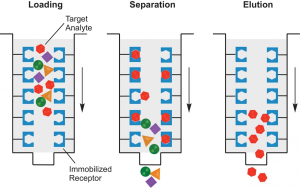
- Purification of ArtB has shown to be difficult in D-galactose and fetuin chromatography. This could be the result of misfolded protein products from the E.coli strain that hosts the plasmid.
Future Plans
- Future research is needed to produce a plasmid that allows for a more concentrated purification of ArtB.
- Determination of cytotoxicity of ArtB subunit
- Development of vaccine using ArtB subunit or ArtAB chimera
Sources
This project was funded by a USDA-NIFA Seed grant to Dr. Richard Beard and Dr. Juliette Tinker (PD Tinker USDA: 2017-05752). We would also like to thank Elise Overgaard, Bradly Morris and Kimberly Dueno for their valuable contributions.
References
- Saitoh, M. Tanaka, K., Nishimori, K., Makino, S., Kanno, T., Ishihara, R., Hatama, S., Kitano, R., Kishima, M., Sameshima, T., Akiba, M., Nakazawa, M., Yokomizo, Y., and I. Uchida. 2005. The artAB genes encode a putative ADP-ribosyltransferase toxin homologue associated with Salmonella enterica serovar Typhimurium DT104. Microbiology 151: 3089-3096
- Tamamura,Y., Tanaka, K., Uchida, I., 2017. Characterization of pertussis-like toxin from Salmonella spp. that catalyzes ADP-ribosylation of G-proteins. Scientific Reports 7:2653-DOI:10.1038/s41598-017-02517-2
Additional Information