Wesley Ercanbrack, Dr. Julia Oxford, Madyson Preston, Dr. Troy Rohn
Abstract
Apolipoprotein-E4 is one of three alleles of the human apolipoprotein-E (APOE) gene; each allele of the gene differs by one amino acid residue-a progressive swap from C112, C158 in ApoE2 to C112, R158 in APOE3, then R112, R158 in APOE4. The APOE4 allele has been recognized over the past 20 years as the largest genetic contributing factor in developing late-onset Alzheimer’s disease (LoAD) whereas the APOE3 allele shows no increase in risk despite only differing by a single amino acid residue. In addition to this relationship with LoAD, inheritance of the APOE4 allele has also been a known contributor to increased risk of cardiovascular disease (CVD). Our current model-zebrafish (Danio rerio)- has been used to identify potential toxic effects of a 151 amino acid amino-terminal protein modeled after a 17kDa ApoE4 protein fragment (nApoE41-151). The nApoE41-151 fragment was identified in human AD post-mortem frontal cortex tissue and has shown to be cytotoxic in both BV2 cell culture and in zebrafish by the Rohn Lab. Our data has indicated that there is a cardiovascular effect during embryogenesis to nApoE41-51 and leads to increased prevalence of enlarged hearts and pericardial edemas. Our project aim is to identify potential cardiovascular actions of nApoE41-51 in zebrafish embryos and to compare these possible effects with a similar nApoE31-151 fragment as a control. This could help identify new treatments and therapies.
Introduction
Alzheimer’s Disease (AD) affects 10% of people who are 65 years old or older in the United States. Cardiovascular Disease affects 48% of all adults in the United States. A suspected link between these two well-known and dangerous diseases is the ApoE4 protein. Everyone has a gene that encodes for the ApoE protein, but depending on each person’s genotype, they may have either the ApoE2, ApoE3, or ApoE4 allele. If an individual has one allele from either parent of the ApoE4 protein, the risk of developing LoAD increases four-fold. If an individual is homozygous for the ApoE4 allele, and inherits one copy from each parent, then the risk of developing LoAD increases ten-fold. The ApoE4 protein in and of itself isn’t dangerous, but it tends to fragment at the 151st amino acid residue. A fragmented protein is no longer able to serve its natural purpose, and it is thought that in the ApoE case, cleavage prevents the protein from removing cholesterol plaques from the cardiovascular system and beta-amyloid plaques from the central nervous system.
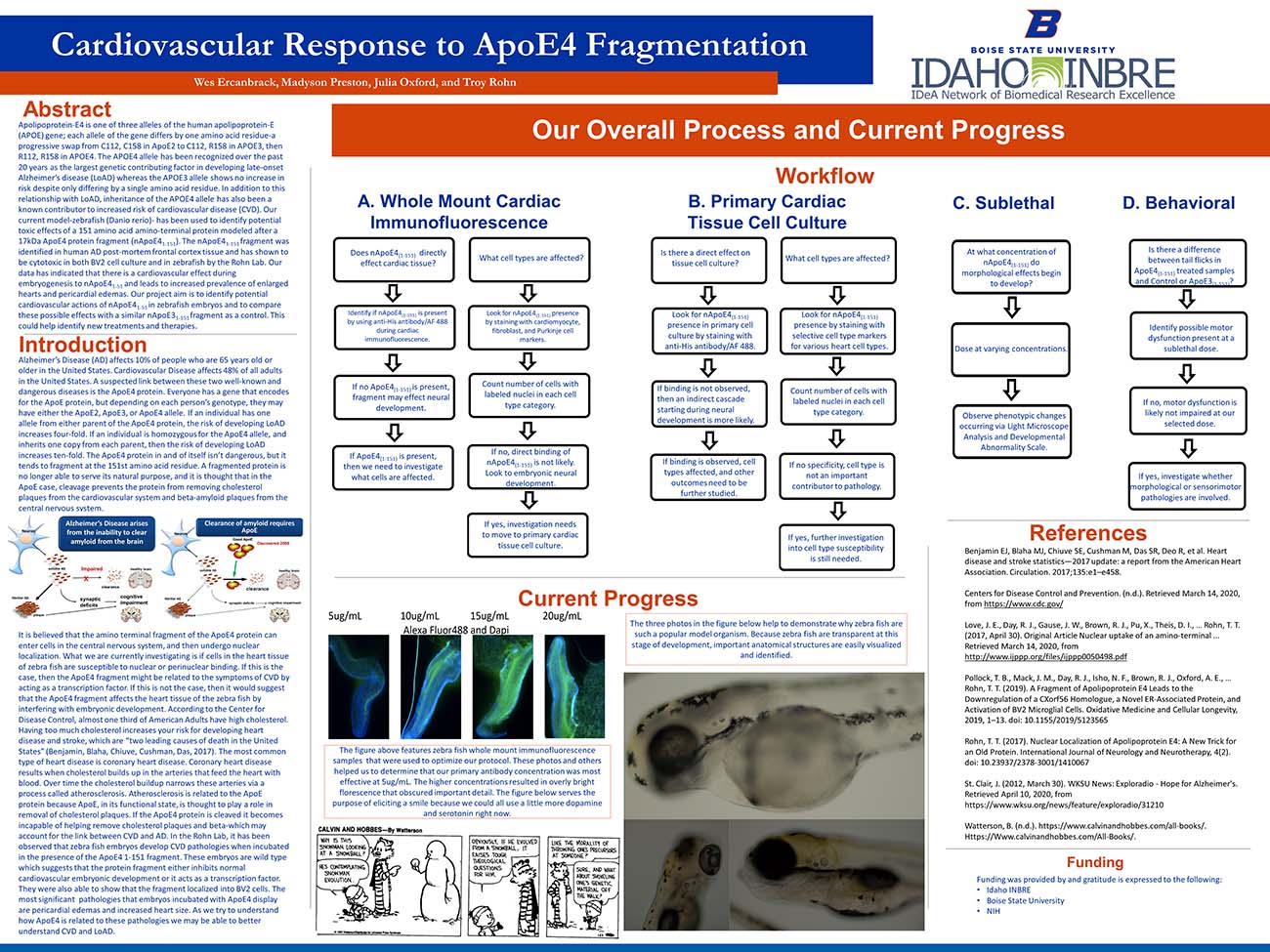
It is believed that the amino terminal fragment of the ApoE4 protein can enter cells in the central nervous system, and then undergo nuclear localization. What we are currently investigating is if cells in the heart tissue of zebra fish are susceptible to nuclear or perinuclear binding. If this is the case, then the ApoE4 fragment might be related to the symptoms of CVD by acting as a transcription factor. If this is not the case, then it would suggest that the ApoE4 fragment affects the heart tissue of the zebra fish by interfering with embryonic development. According to the Center for Disease Control, almost one third of American Adults have high cholesterol. Having too much cholesterol increases your risk for developing heart disease and stroke, which are “two leading causes of death in the United States” (Benjamin, Blaha, Chiuve, Cushman, Das, 2017). The most common type of heart disease is coronary heart disease. Coronary heart disease results when cholesterol builds up in the arteries that feed the heart with blood. Over time the cholesterol buildup narrows these arteries via a process called atherosclerosis. Atherosclerosis is related to the ApoE protein because ApoE, in its functional state, is thought to play a role in removal of cholesterol plaques. If the ApoE4 protein is cleaved it becomes incapable of helping remove cholesterol plaques and beta-which may account for the link between CVD and AD. In the Rohn Lab, it has been observed that zebra fish embryos develop CVD pathologies when incubated in the presence of the ApoE4 1-151 fragment. These embryos are wild type which suggests that the protein fragment either inhibits normal cardiovascular embryonic development or it acts as a transcription factor. They were also able to show that the fragment localized into BV2 cells. The most significant pathologies that embryos incubated with ApoE4 display are pericardial edemas and increased heart size. As we try to understand how ApoE4 is related to these pathologies we may be able to better understand CVD and LoAD.
Our Overall Process and Current Progress
Workflow
A. Whole Mount Cardiac Immunofluorescence
Does nApoE4(1-151) directly effect cardiac tissue?
- Identify if nApoE4(1-151) is present by using anti-His antibody/AF 488 during cardiac immunofluorescence.
- If no ApoE4(1-151) is present, fragment may effect neural development.
- If ApoE4(1-151) is present, then we need to investigate what cells are affected.
What cell types are affected?
- Look for nApoE4(1-151) presence by staining with cardiomyocyte, fibroblast, and Purkinje cell markers.
- Count number of cells with labeled nuclei in each cell type category.
- If no, direct binding of nApoE4(1-151) is not likely. Look to embryonic neural development.
- If yes, investigation needs to move to primary cardiac tissue cell culture.
B. Primary Cardiac Tissue Cell Culture
Is there a direct effect on tissue cell culture?
- Look for nApoE4(1-151) presence in primary cell culture by staining with anti-His antibody/AF 488.
- If binding is not observed, then an indirect cascade starting during neural development is more likely.
- If binding is observed, cell types affected, and other outcomes need to be further studied.
What cell types are affected?
- Look for nApoE4(1-151) presence by staining with selective cell type markers for various heart cell types.
- Count number of cells with labeled nuclei in each cell type category.
- If no specificity, cell type is not an important contributor to pathology.
- If yes, further investigation into cell type susceptibility is still needed.
C. Sublethal
At what concentration of nApoE4(1-151) do morphological effects begin to develop?
- Dose at varying concentrations.
- Observe phenotypic changes occurring via Light Microscope Analysis and Developmental Abnormality Scale.
D. Behavioral
Is there a difference between tail flicks in ApoE4(1-151) treated samples and Control or ApoE3(1-151)?
- Identify possible motor dysfunction present at a sublethal dose.
- If no, motor dysfunction is likely not impaired at our selected dose.
- If yes, investigate whether morphological or sensorimotor pathologies are involved.
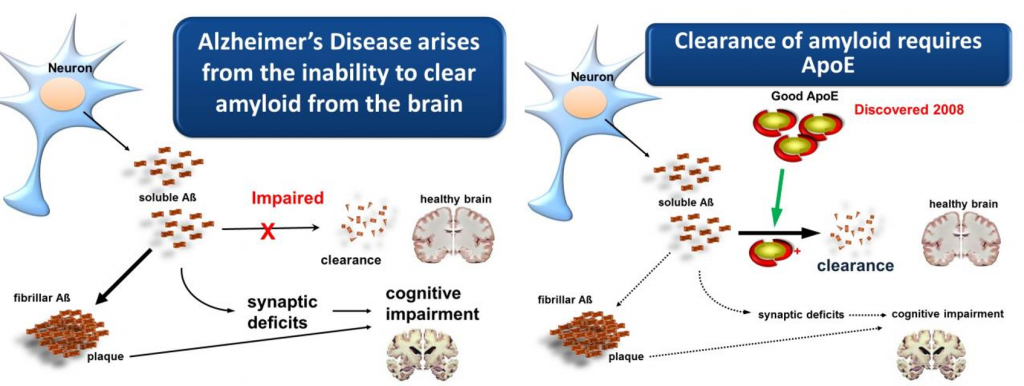
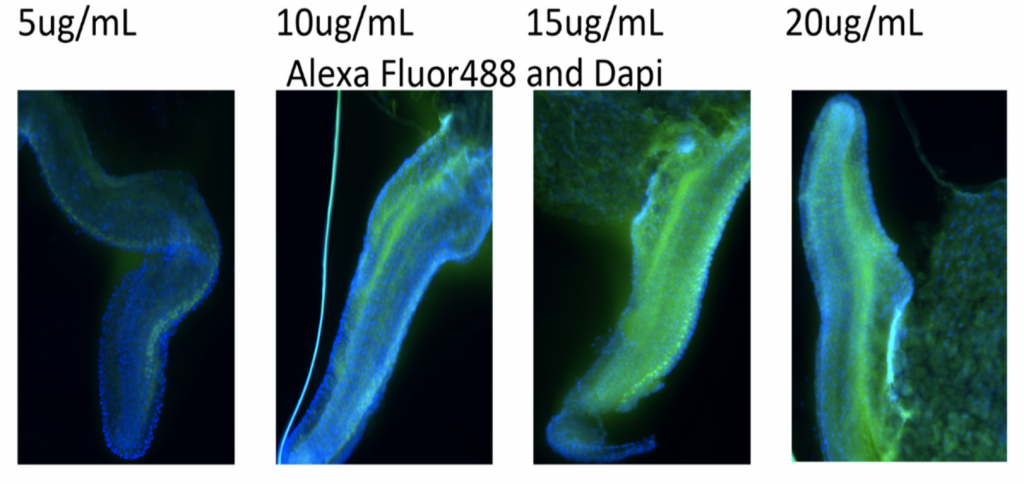
Current Progress
References
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e1–e458.
- Centers for Disease Control and Prevention. (n.d.). Retrieved March 14, 2020, from https://www.cdc.gov/
- Love, J. E., Day, R. J., Gause, J. W., Brown, R. J., Pu, X., Theis, D. I., … Rohn, T. T. (2017, April 30). Original Article Nuclear uptake of an amino-terminal … Retrieved March 14, 2020, from http://www.ijppp.org/files/ijppp0050498.pdf
- Pollock, T. B., Mack, J. M., Day, R. J., Isho, N. F., Brown, R. J., Oxford, A. E., … Rohn, T. T. (2019). A Fragment of Apolipoprotein E4 Leads to the Downregulation of a CXorf56 Homologue, a Novel ER-Associated Protein, and Activation of BV2 Microglial Cells. Oxidative Medicine and Cellular Longevity, 2019, 1–13. doi: 10.1155/2019/5123565
- Rohn, T. T. (2017). Nuclear Localization of Apolipoprotein E4: A New Trick for an Old Protein. International Journal of Neurology and Neurotherapy, 4(2). doi: 10.23937/2378-3001/1410067
- St. Clair, J. (2012, March 30). WKSU News: Exploradio – Hope for Alzheimer’s. Retrieved April 10, 2020, from https://www.wksu.org/news/feature/exploradio/31210
- Watterson, B. (n.d.). https://www.calvinandhobbes.com/all-books/. Https://Www.calvinandhobbes.com/All-Books/.
Funding
Funding was provided by and gratitude is expressed to the following:
- Idaho INBRE
- Boise State University
- NIH
Additional Information
For questions or comments about this research, contact Wesley Ercanbrack at ercanbrack.wesley@gmail.com.