Nathan Harrison, Ryland McDermott, Tyler Smith, LJ McKenzie, Dr. John Thurston, Dr. Danny Xu, Dr. Ken Cornell
Abstract
Giardia is a parasite that causes giardiasis, the most common protozoal intestinal infection reported worldwide that afflicts ~200 million people annually. While not a fatal disease, studies have shown that children are more likely to be infected than adults and can suffer malnutrition and reduced growth rates. Currently, Giardia infections are typically treated with only nidazole drugs such as metronidazole (MTZ). With the rise of antibiotic resistance in all parts of the world, new drugs are desperately needed to combat even common infectious diseases. 5’ Methylthioadenosine nucleosidase (MTN), a critical component of the S-Adenosylhomocysteine (SAM) pathway that plays an important role in adenine salvage that is essential for Giardia replication and growth. The purpose of this project is to develop antiparasitic medications that could combat Giardia by interrupting MTN activity. Further, we demonstrate that a number of MTN inhibitors show synergistic activity with MTZ. Thus, these compounds may be useful in combating MTA resistance that is emerging for Giardia infections in humans and animals.
Background
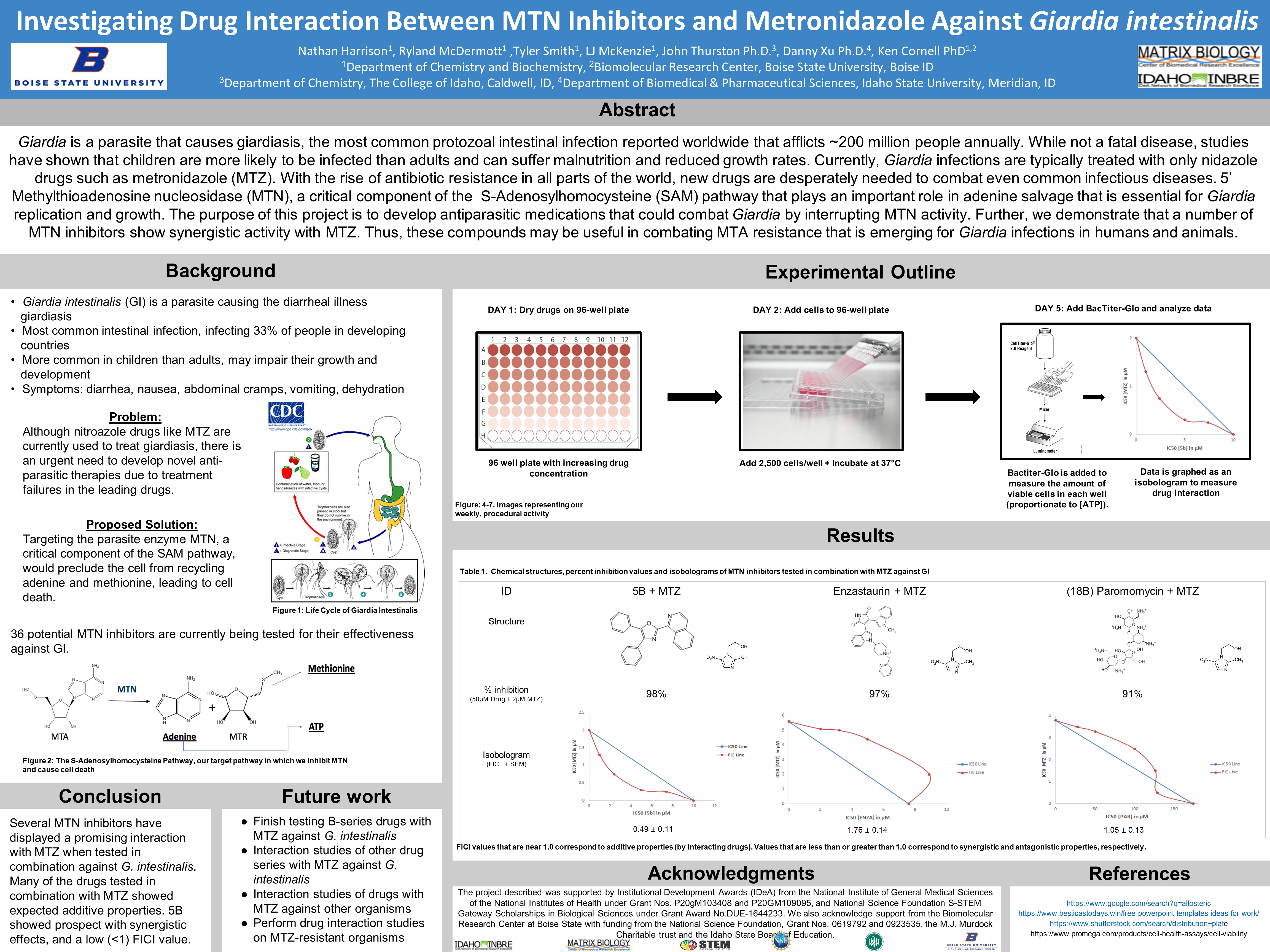
- Giardia intestinalis (GI) is a parasite causing the diarrheal illness giardiasis
- Most common intestinal infection, infecting 33% of people in developing countries
- More common in children than adults, may impair their growth and development
- Symptoms: diarrhea, nausea, abdominal cramps, vomiting, dehydration
Problem:
Although nitroazole drugs like MTZ are currently used to treat giardiasis, there is an urgent need to develop novel anti-parasitic therapies due to treatment failures in the leading drugs.
Proposed Solution:
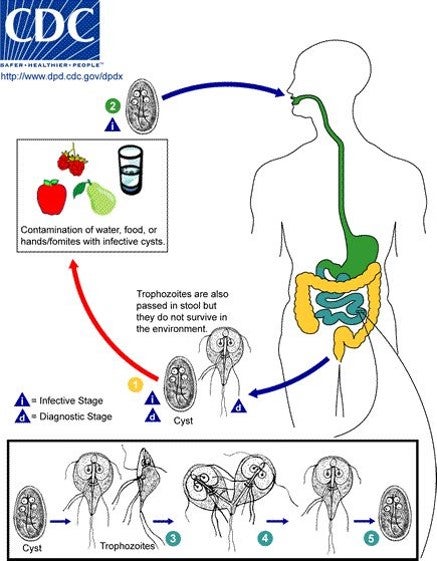
Targeting the parasite enzyme MTN, a critical component of the SAM pathway, would preclude the cell from recycling adenine and methionine, leading to cell death.
36 potential MTN inhibitors are currently being tested for their effectiveness against GI.
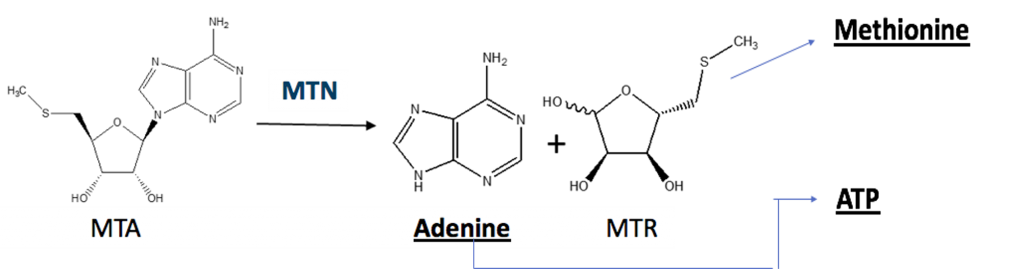
Experimental Outline
Results
Conclusion
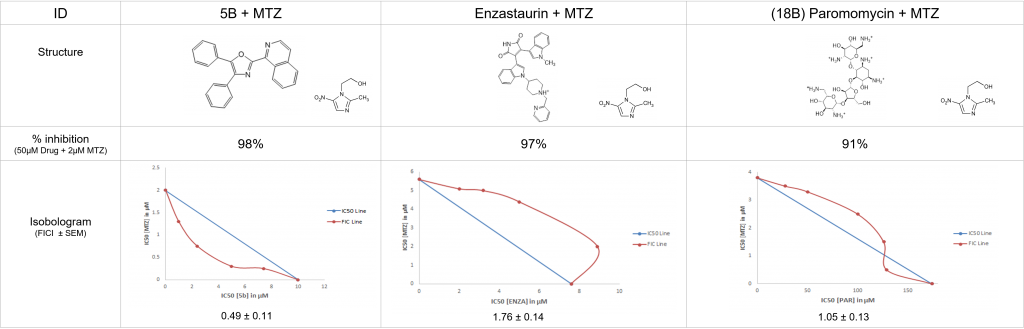
Several MTN inhibitors have displayed a promising interaction with MTZ when tested in combination against G. intestinalis.
Many of the drugs tested in combination with MTZ showed expected additive properties. 5B showed prospect with synergistic effects, and a low (<1) FICI value.
Future Work
- Finish testing B-series drugs with MTZ against G. intestinalis
- Interaction studies of other drug series with MTZ against G. intestinalis
- Interaction studies of drugs with MTZ against other organisms
- Perform drug interaction studies on MTZ-resistant organisms
Acknowledgements
The project described was supported by Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant Nos. P20gM103408 and P20GM109095, and National Science Foundation S-STEM Gateway Scholarships in Biological Sciences under Grant Award No.DUE-1644233. We also acknowledge support from the Biomolecular Research Center at Boise State with funding from the National Science Foundation, Grant Nos. 0619792 and 0923535, the M.J. Murdock Charitable trust and the Idaho State Board of Education.
References
- https://www.google.com/search?q=allosteric
- https://www.besticastodays.win/free-powerpoint-templates-ideas-for-work/
- https://www.shutterstock.com/search/distribution+plate
- https://www.promega.com/products/cell-health-assays/cell-viability
Additional Information
For questions or comments about this research, contact Nathan Harrison at nathanharrison@u.boisestate.edu.