Zoe Hutchinson, Gamid Abatchev, Andrew Bogard, Jason Ward, Dr. Daniel Fologea
Abstract
Liposomes are self-enclosed spherical lipid bilayer membranes that present a great potential for drug delivery. Liposome preparation and loading may be achieved by several methods methods including extrusion and sonication. These methods can be time consuming, require specific equipment, and of require further steps to purify produced liposomes of loaded contents. To alleviate this problem, we propose using electrodialysis as a fast technique for liposome preparation. Our approach is based on their electrophoretic movement when exposed to an electric field. We subjected homogeneous solutions of lipids and ionic detergents to electrodialysis through a polycarbonate membrane. The migration of the detergent out of the mixture allows the lipids to self-assemble into liposomes, incorporating designated drug/drug simulator molecules present in the initial mixture. The obtained liposomes were characterized by Dynamic Light Scattering (DLS), microscopy, and fluorescence spectroscopy (FS). Our results show that the procedure may also be applied for preparation of liposomes capable of evading the immune system, therefore presenting potential for use as drug carriers and in vivo applications with higher efficacy than traditional liposomes.
Objectives
- Prepare liposomes using electrodialysis (ED) techniques and ionic detergents
- Prepare and load liposomes concurrently with drug simulating dyes
- Characterize the prepared liposomes (e.g size, payload incorporation) using microscopy, DLS, and FS
Materials and Methods
Materials:
Lipids:
Asolectin (Aso), Cholesterol (Chol), 1,2-distearoyl-sn-glycero-3 phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamineN-[methoxy(polyethylene glycol)-2000] (DSPE-PEG)
Compositions:
A – 5 mg Aso:2 mg Chol
B – 4.1 mg DSPC:1.9 mg Chol:1.3 mg DSPE-PEG
Buffers:
135 mM KCl + 20 HEPES, 20 mM KCl + 5 mM HEPES
Detergents Used:
Cholic Acid 10% W/V (CA), Sodium dodecyl sulfate 10% W/V (SDS)
Dyes:
Rhodamine (Rdn) and Acridine Orange (AO).
Detergent for Release:
Detergent For Release: Triton X-100 5% v/v (TX)
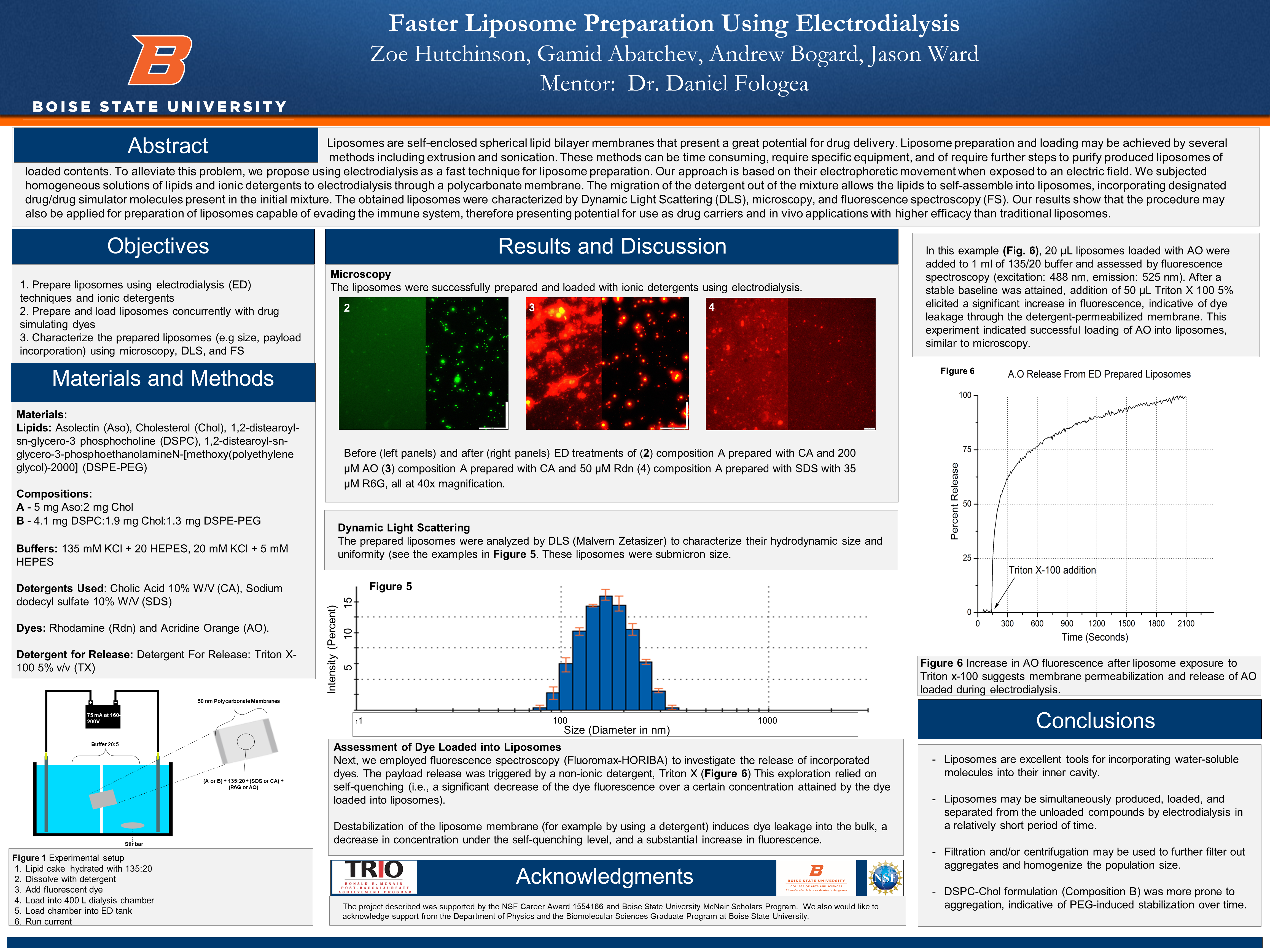
Lipid cake hydrated with 135:20
Dissolve with detergent
Add fluorescent dye
Load into 400 L dialysis chamber
Load chamber into ED tank
Run current
Results and Discussion
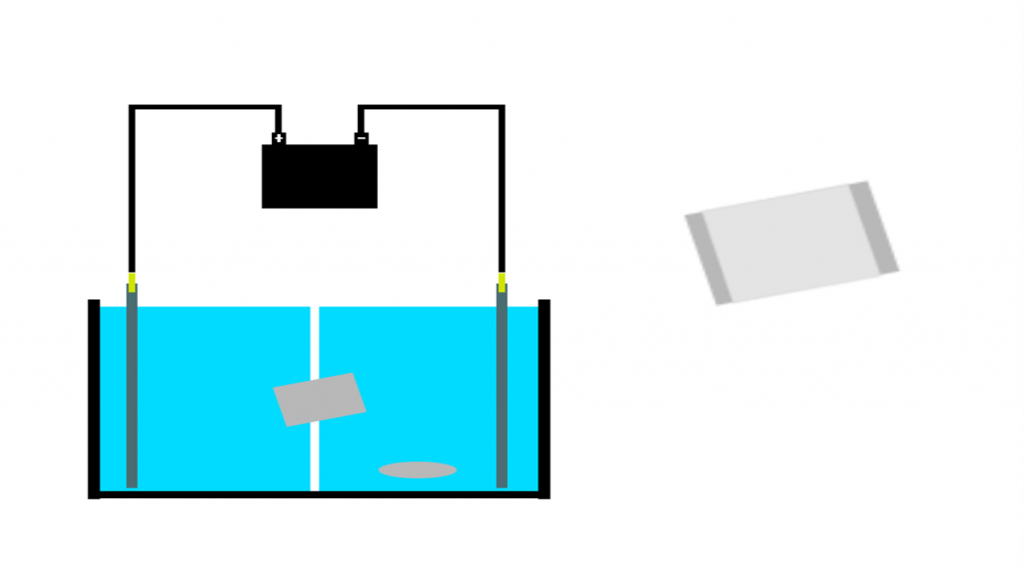
Microscopy
The liposomes were successfully prepared and loaded with ionic detergents using electrodialysis.
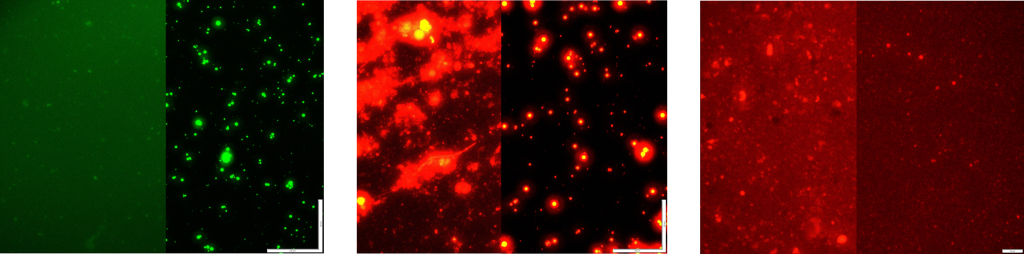
Dynamic Light Scattering
The prepared liposomes were analyzed by DLS (Malvern Zetasizer) to characterize their hydrodynamic size and uniformity (see the examples in Figure 5. These liposomes were submicron size.
Assessment of Dye Loaded into Liposomes
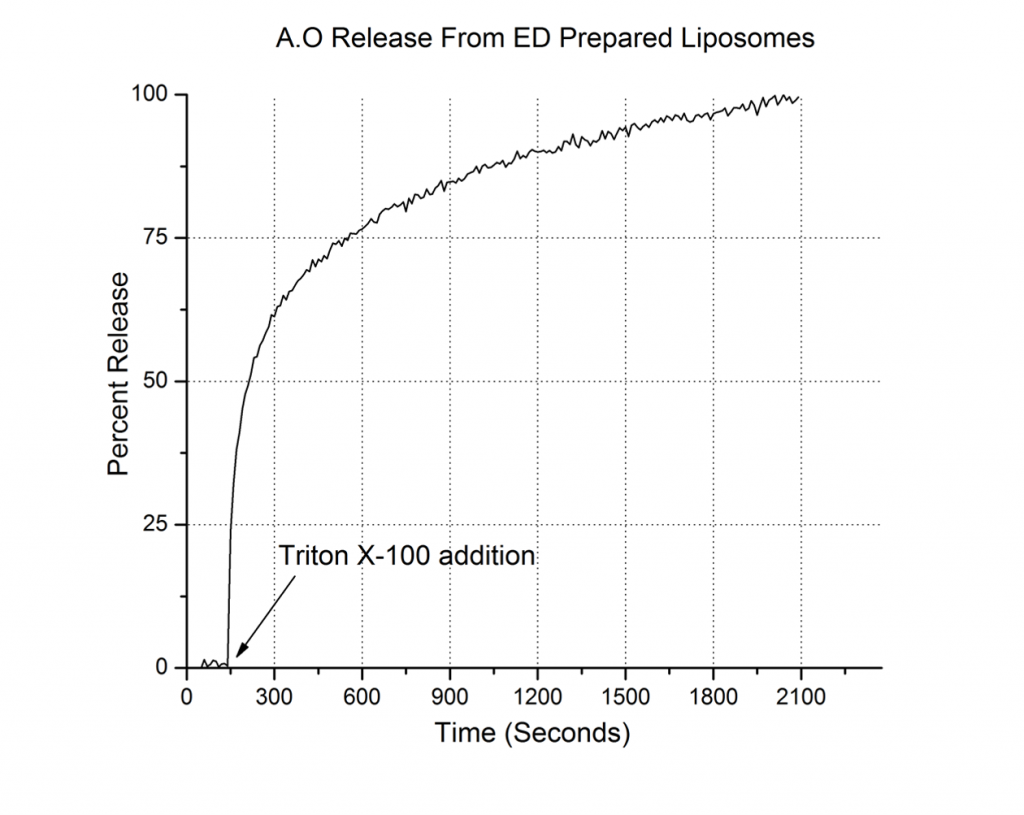
Next, we employed fluorescence spectroscopy (Fluoromax-HORIBA) to investigate the release of incorporated dyes. The payload release was triggered by a non-ionic detergent, Triton X (Figure 6) This exploration relied on self-quenching (i.e., a significant decrease of the dye fluorescence over a certain concentration attained by the dye loaded into liposomes).
Destabilization of the liposome membrane (for example by using a detergent) induces dye leakage into the bulk, a decrease in concentration under the self-quenching level, and a substantial increase in fluorescence.
In this example (Fig. 6), 20 μL liposomes loaded with AO were added to 1 ml of 135/20 buffer and assessed by fluorescence spectroscopy (excitation: 488 nm, emission: 525 nm). After a stable baseline was attained, addition of 50 μL Triton X 100 5% elicited a significant increase in fluorescence, indicative of dye leakage through the detergent-permeabilized membrane. This experiment indicated successful loading of AO into liposomes, similar to microscopy.
Conclusions
- Liposomes are excellent tools for incorporating water-soluble molecules into their inner cavity.
- Liposomes may be simultaneously produced, loaded, and separated from the unloaded compounds by electrodialysis in a relatively short period of time.
- Filtration and/or centrifugation may be used to further filter out aggregates and homogenize the population size.
- DSPC-Chol formulation (Composition B) was more prone to aggregation, indicative of PEG-induced stabilization over time.
Acknowledgements
The project described was supported by the NSF Career Award 1554166 and Boise State University McNair Scholars Program. We also would like to acknowledge support from the Department of Physics and the Biomolecular Sciences Graduate Program at Boise State University.
Additional Information
For questions or comments about this research, contact Zoe Hutchinson at zoehutchinson@u.boisestate.edu.