
Professor, Department of Biological Sciences
Year arrived at BSU: 2000
Mailing Address:
Department of Biology
Boise State University
Boise, ID 83725-1515
Office Location: Science Building, Room 117
Office Number: 208-426-2396
Lab Location: Science Building, Room 216
E-Mail Address: trohn@boisestate.edu
Academic Degrees
Troy Rohn graduated in 1990 from the University of California at Davis with a B.S. in Physiology. He received his Ph.D. in Pharmacology from the University of Washington, Seattle in 1994. His interests include the role of ApoE4 in Alzheimer’s disease. Dr. Rohn had several Postdoctoral stints including two-plus years living in Paris, France, one year at Montana State University in Bozeman, Montana, and two years at UC Irvine. He has obtained extramural funding continuously since his arrival at BSU including grants from NIH, AFAR, and AHAF. Dr. Rohn recently was awarded a 3-year renewal of his NIH NIA R15 grant he has held since 2013.
Teaching
BIOL 442: This is a molecular neurobiology course for undergraduate and graduate students. Topics covered are all aspects of neuronal function at the molecular level. A discussion of several neurodegenerative diseases including Parkinson’s, Alzheimer’s, and Schizophrenia are just a few of the diseases covered.
BIOL 431: This is a general pharmacology course for undergraduate and graduate students. Topics include pharmacokinetics and pharmacodynamics. All major drug classes are covered in this course including drugs that affect the heart, brain, vasculature, and all other major organ systems.
BIOL 100 at Boise State University (Electronic): This is a non-major course covering all aspects of biology (taught online in the summers).
BIOL 320: A cell biology course that represents a core requirement for all biology majors.
UF 100: Brain Matters: A survey of the brain covering behavior, plasticity, genetics, and many diseases and disorders.
BIOL 117: Introduction to Neuroscience Through Disease Models
RESEARCH INTERESTS
The Rohn lab has a long-standing interest in understanding the molecular underpinnings of Alzheimer’s disease (AD). During the progression of Alzheimer’s disease, many brain cells die particularly in the area of the hippocampus. Because the hippocampus is an area of the brain involved in memory, AD is primarily a disease where afflicted individuals lose their capacity for memory and eventually other important cognitive skills involved in executive functions.
The primary focus of my lab currently is understanding how inheritance of the apolipoprotein E4 (APOE4) gene greatly enhances dementia risk. Although it is well established that inheritance of the APOE4 allele increases the risk of AD approximately tenfold, the mechanism of how this protein contributes to AD pathogenesis remains unknown. We have recently discovered that a fragment of ApoE4 in the human AD brain when produced is toxic and inflammatory.
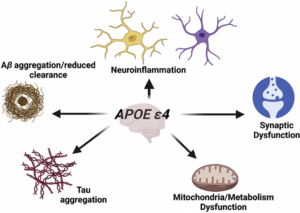
We are excited to currently be examining the in vivo effects of ApoE fragments in zebrafish a new model system for the lab where we have recently shown that following treatment of embryos with an amino-terminal fragment of ApoE4, leads to toxicity and effects on the heart including a significant decrease in heart rate. We have also generated a mutant zebrafish strain that specifically expresses this ApoE4 fragment and are in the process of characterizing these fish through molecular techniques and behavioral analyses.
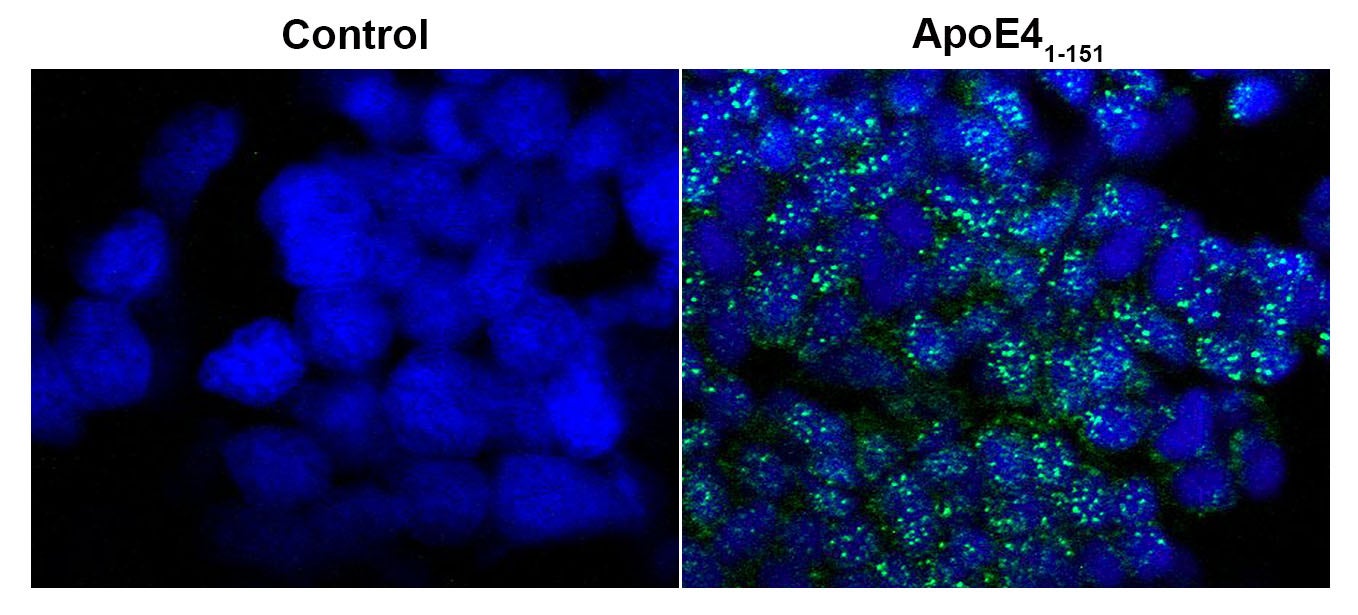
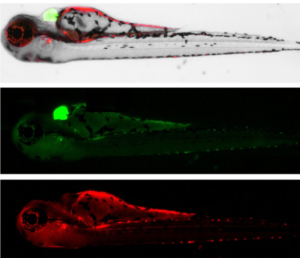
Figure showing the expression of an amino-terminal fragment of ApoE4. Representative expression of mCherry (red) in the brain and notochord following injection into one-cell stage zebrafish. The expression of enhanced green fluorescent protein under the cardiac myosin light chain 2 promoter (green), allows for easy screening of zebrafish for the mutant ApoE4 gene. These pictures were obtained by a current undergraduate student, Alex LaFollette who is working in the lab as a B2B student.
RECENT PUBLICATIONS (selected from 80 total)
H-index 40
Hall, S.E., White, Z.J., Rohn, T.T., Sudasinghe, K.H. and Young, M.E. (2025). Behavioral time course of exercise neuroprotection in male and female TgF344-AD rats. Behavioral Brain Research, (In Revision).
Troy T. Rohn. Embracing a new era in medicine through gene therapy. The Academic, August 27, 2024
Troy T. Rohn, Dean Radin, Tracy Brandmeyer, Peter G. Seidler, Barry J. Linder, Tom Lytle, John L. Mee and Fabio Macciardi. (2024). Treatment with shRNA to Knockdown the 5-HT2A Receptor Improves Memory In Vivo and Decreases Excitability in Primary Cortical Neurons. Genomic Psychiatry. https://doi.org/10.61373/gp024r.0043.
Troy T. Rohn, Dean Radin, Tracy Brandmeyer, Peter G. Seidler, Barry J. Linder, Tom Lytle, John L. Mee and Fabio Macciardi. (2024). Intranasal Delivery of shRNA to Knockdown the 5HT-2A receptor Enhances Memory and Alleviates Anxiety (2024). Translational Psychiatry: Published 20 March 2024. Volume 14: 154.
Troy T. Rohn and Dean Radin (2024). Long-lasting genetic therapeutics for debilitating neurological conditions. Nature Biopharmdeal, March 2024.
Troy T. Rohn, Dean Radin, Tracy Brandmeyer, Barry J. Linder, Emile Andriambeloson, Stéphanie Wagner, James Kehler, Ana Vasileva, Huaien Wang, John L. Mee and James H. Fallon. (2023). Genetic modulation of the HTR2A gene reduces anxiety-related behavior in mice. PNAS Nexus, Volume 2, Issue 6, June 2023, pgad170, https://doi.org/10.1093/pnasnexus/pgad170
McCarthy, M.M., Hardy, M.J., Leising, S.E., LaFollette, A., Stewart, E.S., Cogan, A.S., Sanghal, T., Matteo, K., Oxford, J.T., and Rohn T.T. (2022). An amino-terminal fragment of apolipoprotein E4 leads to behavioral deficits, increased PHF-1 immunoreactivity, and mortality in zebrafish. PLOS One Dec 15;17(12):e0271707. doi: 10.1371/journal.pone.0271707. eCollection 2022
RESEARCH OPPORTUNITIES
The lab is not accepting any positions for the 2025-26 academic year for both undergraduate and graduate students
OTHER ACTIVITIES
Associate Editor
Associate Editor for the scientific journal, Frontiers in Dementia
Associate Editor for the scientific journal, Frontiers in Aging Neuroscience