About Me:
Associate Professor
Organic & Inorganic Chemistry
Email: ericbrown3@boisestate.edu
Office: SCNC 314
Phone: (208) 426-1186
Research Interests:
- Biological and Inorganic Chemistry
- Bioinspired Catalysis
- Organometallic Chemistry
- Chemical Education
Additional Information:
Undergraduate Research Assistant
What am I looking for in an undergraduate research assistant?
What courses would you recommend students have taken prior to working in your laboratory?
- CHEM 112 (General Chemistry II) – Minimum
- CHEM 307 (Organic Chemistry I) – Preferred
How many hours per week do you expect a student to spend in the laboratory per research credit and does the time have to be a set schedule?
- I expect my research students to come in twice per week for a total of 8-10 hrs per week.
Ideally, for what length of time would a student research in your laboratory to achieve a meaningful research experience?
- The minimum length of time for a student to achieve a meaningful research experience is two semesters.
Educational Background
2006: NIH Postdoctoral Fellow | University of Minnesota
2002: Ph.D. | Oregon State University
1997: B.S. | University of Idaho
Research Overview
Proteins containing transition metal ions carry out a variety of important functions in biological systems. Consequently, understanding how these chemical transformations occur and what factors regulate their reactivity is an important research area. The focus of our research is to provide fundamental insight into the structure and function of metalloenzymes by using the synthetic modeling approach. The synthetic modeling approach involves the synthesis of low molecular weight complexes that model the structural and functional units of the enzymes. Useful information such as spectroscopic and structural data and identification of possible intermediates or pathways in the enzymatic cycle can be obtained through studies of the synthetic model complexes.
Students involved in research in our laboratory will obtain multidisciplinary training in the synthesis of organic and inorganic compounds. They will also be exposed to a variety of characterization methods that are used to characterize inorganic compounds and to examine their reactivity. These include X-ray crystallography and NMR, IR, UV-Vis, and EPR spectroscopies. Finally, students will develop an understanding of mechanism by examining the reactivity and kinetics of their model complexes with either natural or model substrates.
Below are examples of different bioinorganic projects we are currently pursuing in our laboratory.
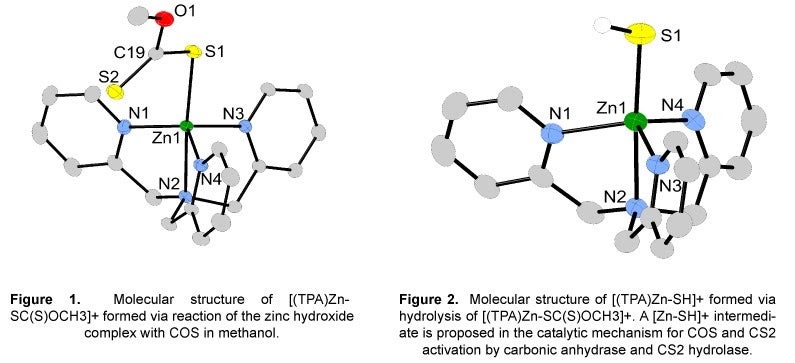
I. Activation of Sulfur-Containing Heterocumulenes:
Emissions of carbon dioxide (CO2) and carbonyl sulfide (COS) are expected to increase within the next century and could have a significant impact on the earth’s climate. Consequently, the development of a practical method for converting CO2 and COS into more useful compounds is recognized to be important for a sound long-term energy policy. Our research focuses on the synthesis and structural definition of new bio-inspired inorganic compounds useful for the activation of sulfur-containing heterocumulenes, such as COS and CS2. The inspiration behind the design features of our inorganic compounds is carbonic anhydrase and CS2 hydrolase. Studies have shown carbonic anhydrase in plants, algae and lichens and CS2 hydrolase in thermophilic archaea react with COS and CS2, respectively, to give hydrogen sulfide (H2S). Studies directed at understanding the mechanism of COS and CS2 activation by carbonic anhydrase and CS2 hydrolase has been very limited and is the impetus for this research.
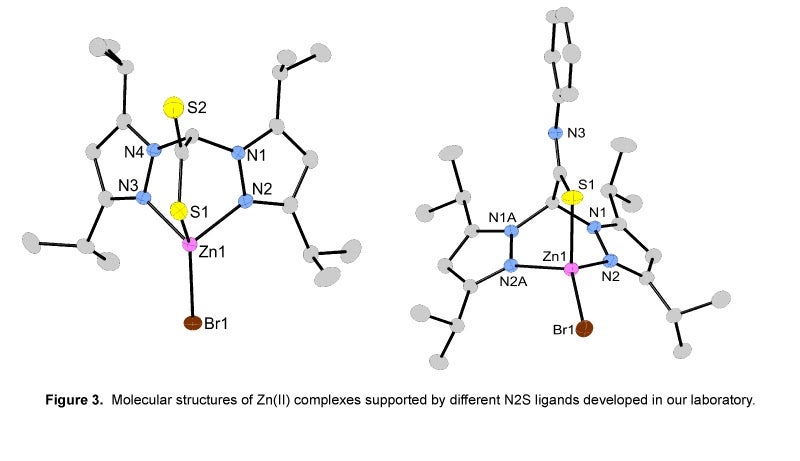
II. Preparation of Metal Complexes Containing N2S Donor Atom Sets of Relevance to Peptide Deformylase:
The function of the metalloenzyme peptide deformylase (PDF) is to deformylate proteins that contain N-formylated methionine. Since this deformylation sequence is unique to prokaryotes, understanding the detailed mechanism of PDF may be important in the development of future antibiotics. In addition, further motivation for studying the mechanism comes from the unusual finding that the most active form of PDF is not a zinc-containing enzyme but instead an iron-containing enzyme. To gain insight into the unusual metal-dependent reactivity of PDF, our research is focused on the design, synthesis and mechanistic evaluation of FeII and ZnII hydroxide complexes supported with novel N2S ligand systems. These N2S ligand systems model the cysteine and two histidine binding motif present in the active site of PDF.
Select Publications (2018-2021)
Shin, D.; Gorgulla, C.; Boursier, M. E.; Rexrode, N.; Brown, E. C.; Arthanari, H.; Blackwell, H. E.; Nagarajan, R. “N-Acyl Homoserine Lactone Analog Modulators of the Pseudomonas aeruginosa Rhll Quorum Sensing Signal Synthase,” ACS Chemical Biology 2019, 14, 2305-2314.
Millard, S.; Fothergill, J. W.; Anderson, Z.; Brown, E. C.; King, M. D.; Colson, A. C.
“Supramolecular Interactions of Group VI Metal Carbonyl Complexes: The Facilitating Role of 1,3-Bis(p-isocyanophenyl)urea,” Inorganic Chemistry 2019 58 (12), 8130-8139.
Boursier, M. E.; Moore, J. D.; Heitman, K. M.; Shepardson-Fungairino, S. P.; Combs, J. B.; Koenig, L. C.; Shin, D.; Brown, E. C.; Nagarajan, R.; Blackwell, H. E. “Structure-Function Analyses of the N-Butanoyl L-Homoserine Lactone Quorum-Sensing Signal Define Features Critical to Activity in RHIR,” ACS Chem. Biol. 2018, 13, 2655–2662.
Lam, M. N.; Dudekula, D.; Durham, B.; Collingwood, N.; Brown, E. C.; Nagarajan, R. “Insights into β-Ketoacyl-Chain Recognition for β-Ketoacyl-ACP Utilizing AHL Synthases,” Chem. Commun. 2018, 54, 8838-8841.
Tran, V.; Allen, K. E.; Garcia Chavez, M.; Aaron, C.; Dumais, J. J.; York, J. T.; Brown, E. C. “Solution and Solid-State Characterization of Zn(II) Complexes Containing a New Tridentate N2S Ligand,” Polyhedron 2018, 147, 131-141.
Elsberg, J. G.; Spiropulos, N.G.; Colson, A. C.; Brown, E. C. “Crystal Structure of a Homoleptic Zinc(II) Complex Based on Bis(3,5-diiso-propyl-pyrazol-1-yl)acetate,” Acta Crystallogr E Crystallogr Commun. 2018, 74, 1259-1262.
Select Grants (2018-2021)
9/1/2020 – 8/31/2021
- A PriSM for Origin of Life Chemistry – U of I flow thru
Teaching
- CHEM 301 Survey or Orgnic Chemistry
- CHEM 307 Organic Chemistry I
- CHEM 309 Organic Chemistry II
- CHEM 323 Advanced Synthesis Laboratory
- CHEM 401/501 Advanced Inorganic Chemistry
Patents
Strong, A., Brown, E. C., Lee, J., & Graugnard, E. (2018). Transition Metal Guanidinates and Heteroleptic Compounds for Chemical Vapor Deposition and Atomic Layer Deposition. United States. [Intellectual Property Type: Patent – Provisional]