Drake Sanborn, Amanda White, Michal Okebiorun, Adam Croteau, Jacob Tenorio, Dr. Ken Cornell, Dr. Don Plumlee, Dr. Jim Browning

Introduction
- CAP plasma scalpel delivers plasma capable of killing bacteria found in chronic wounds
- Scalpel used to selectively treat only stained necrotic cells
- Prior research shows that CAP plasma can reduce bacterial colonies by 50% in 10 s and 90% after 30 s depending on substrate surface
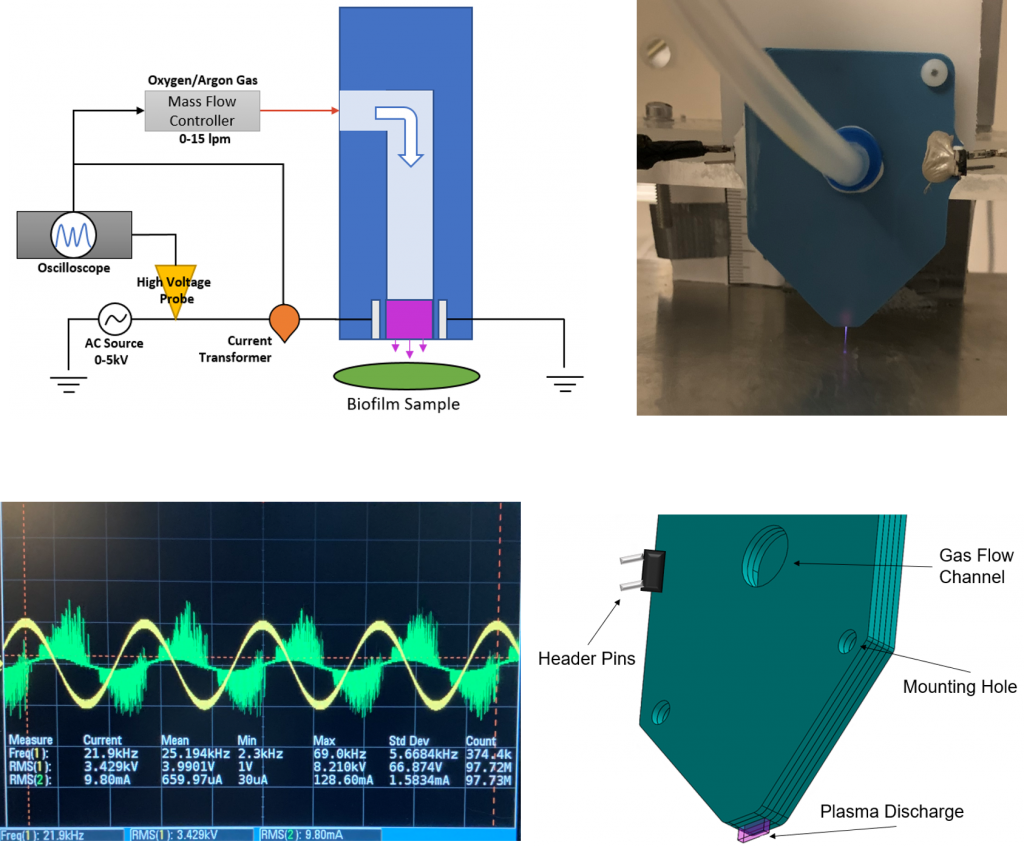
CAP Scalpel Description
- Scalpel fabricated from Low Temperature Co-Fired Ceramic (LTCC)
- AC electrodes embedded under 35 µm of dielectric
- Gas flow channel between plates for Ar
- Discharge operates at 21kHz and 2-5 kV
Plasma Scalpel Apparatus
Experimental Approach
Typical Plasma exposure parameters
- Time: 2 – 5 min
- Voltage: 2.0 – 5.0 kV
- Frequency: 20kHz
- Ar flow: 3 lpm
- Dreschel Flask bubbler with DI water
Biofilms and substrates studied
- Pseudomonas fluorescens
- Escherichia coli 0157:H7 (ATCC 43894)
- Glass coverslips
- Steel coverslips
Biofilm Preparation
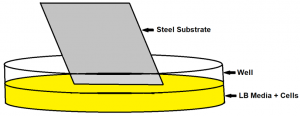 Biofilms prepared by inoculating 12-well plates with 1:100 diluted overnight stocks
Biofilms prepared by inoculating 12-well plates with 1:100 diluted overnight stocks- Substrates inserted vertically into the wells, then placed in incubator
- Coverslips removed from incubator, briefly dipped in DI water, then treated with plasma
Profilometry
Biofilm thickness measured using Stylus Profilometer
Typical Biofilm Heights
- Pseudomonas fluorescens : 3 – 10 µm
- Escherichia coli: 1 – 3 µm
Trypan Blue Staining Process
 Biofilm slides fully submerged in formalin to fix biofilm to the substrate
Biofilm slides fully submerged in formalin to fix biofilm to the substrate- Excess formalin removed and trypan blue stain (0.4%) added to slides
- Only dead cells stained with trypan blue and biofilm region is clearly visible
Image Processing
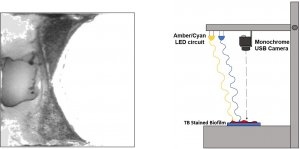 Stained slides set onto the center of XY stage, below monochrome camera
Stained slides set onto the center of XY stage, below monochrome camera- LEDs used to provide peak reflectance and peak absorbance of trypan blue stain
- Amber LED (λ=580 nm) provides peak absorbance
- Cyan LED (λ=495 nm) provides peak reflectance
- Differential taken between Cyan and Amber images to create single composite image
- MATLAB processes image with thresholding to detect edges and display uniform biofilm region
Results
Biofilm Staining Results
- Most biofilm slides are able to be stained with TB
- The slides are dark and show all of the biofilm region, making imaging easier
Plasma Scalpel Etch Results
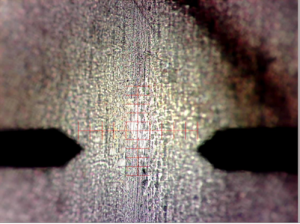 One successful test that showed etching with the plasma scalpel was conducted for 5 min at 2.2kV and a proximity of 5 mm
One successful test that showed etching with the plasma scalpel was conducted for 5 min at 2.2kV and a proximity of 5 mm- Scalpel removed 1 – 2 µm of biofilm and began to expose steel substrate below
Current Issues
- Producing consistent biofilm samples
- Better gas flow control needed
Future Work
Begin testing on tissue and tissue-like materials
- Pig’s ear from live pig
- Matrigel, a gelatinous protein substrate
Create autonomous program that images a stained slide and selectively treats biofilm regions with plasma scalpel
Acknowledgements
This research is supported by the U.S. Department of Agriculture under the NIFA grant # 2018-67018-27881, by the National Institutes of Health (NIH) under Grant # 1R15EB024930-01A1, and program grants NIH/NIGMS P20GM103408 and P20GM109095. The project is also supported by the Helmsley Charitable Trust and Boise State University College of Innovation and Design as a Vertically Integrated Projects course in Plasma Medicine.
Additional Information
For questions or comments on this research, contact Drake Sanborn at drakesanborn@u.boisestate.edu.