Noah Guidara, James Lamb, Dr. Marcus Pearlman, Attila Rektor, Dr. Nirmala Kandadai
Introduction
Infrared thermography looks at images generated by light emissions within the infrared spectrum, specifically those caused by heat to analyze and draw conclusions. One of the FLAIR Lab’s projects at Boise State University is using a powerful laser to heat up a designated substrate and measure how the infrared radiation affects it in both time and space. This study pertains to the notion of black-body radiation, where an object absorbs all incoming light and emits only heat. Because heat emission and transmission varies based on density, conductivity, surface area, distance from light source, and other factors, infrared thermography can be used to detect hidden edges, cracks, mounds, and troughs on a substrate.
Premise
A biofilm is a thin grouping of microorganisms that accumulate on organic material. Some examples include residue within a milk carton, dental plaque, and large biofilms on top of hot springs. Such biofilms applied onto a base material would both absorb and diffuse the energy of a laser differently than that of its base substrate. Because a biofilm is a living substance, its individual components are connected with an extracellular matrix with intertwines the entire mass. This complex relationship means that infrared readings measuring both ambient emission and emissions when a laser is applied will vary significantly from that of a material such as a metal or inorganic compound.
Applications
In a medical setting, such biofilm imaging and detection could be used to determine precise boundaries of bacterial infection inside a wound, which a laser could remove via ablation. In a factory setting, if a biofilm accumulates somewhere within a pipe or container and production would have to shut down to detect this, imaging of a biofilm where the biofilm is on the back side of the material may allow for a determination to be made about its presence if the infrared images are significantly different than that of a material without a biofilm inside.
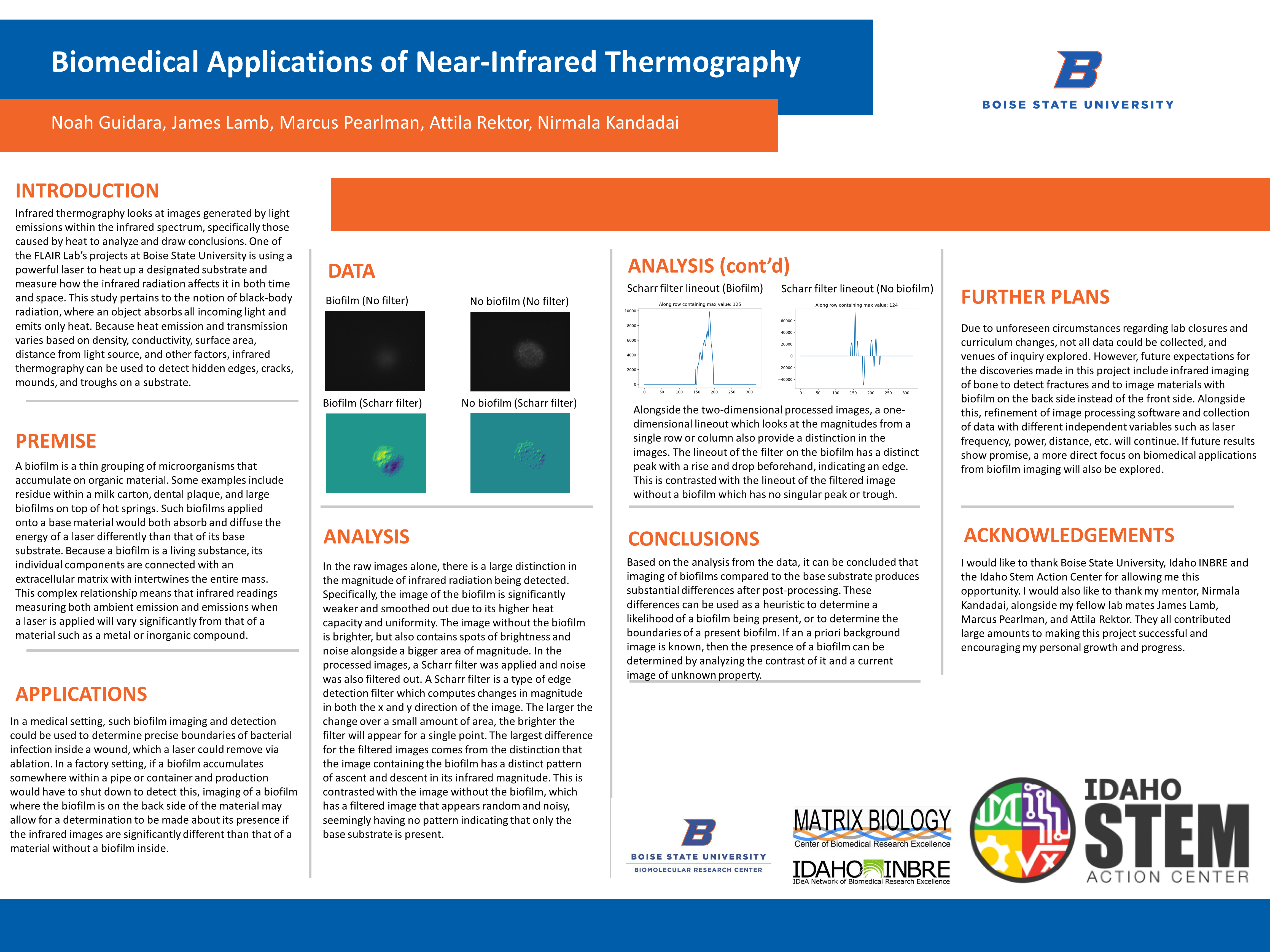
Data – Raw Images
Analysis
In the raw images alone, there is a large distinction in the magnitude of infrared radiation being detected. Specifically, the image of the biofilm is significantly weaker and smoothed out due to its higher heat capacity and uniformity. The image without the biofilm is brighter, but also contains spots of brightness and noise alongside a bigger area of magnitude. In the processed images, a Scharr filter was applied and noise was also filtered out. A Scharr filter is a type of edge detection filter which computes changes in magnitude in both the x and y direction of the image. The larger the change over a small amount of area, the brighter the filter will appear for a single point. The largest difference for the filtered images comes from the distinction that the image containing the biofilm has a distinct pattern of ascent and descent in its infrared magnitude. This is contrasted with the image without the biofilm, which has a filtered image that appears random and noisy, seemingly having no pattern indicating that only the base substrate is present.
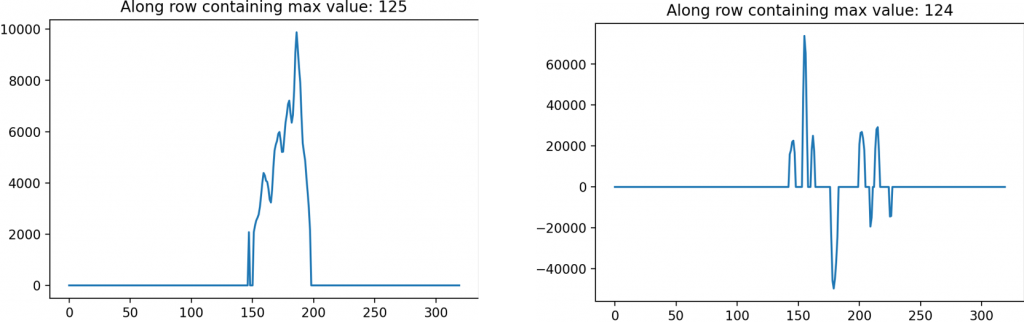
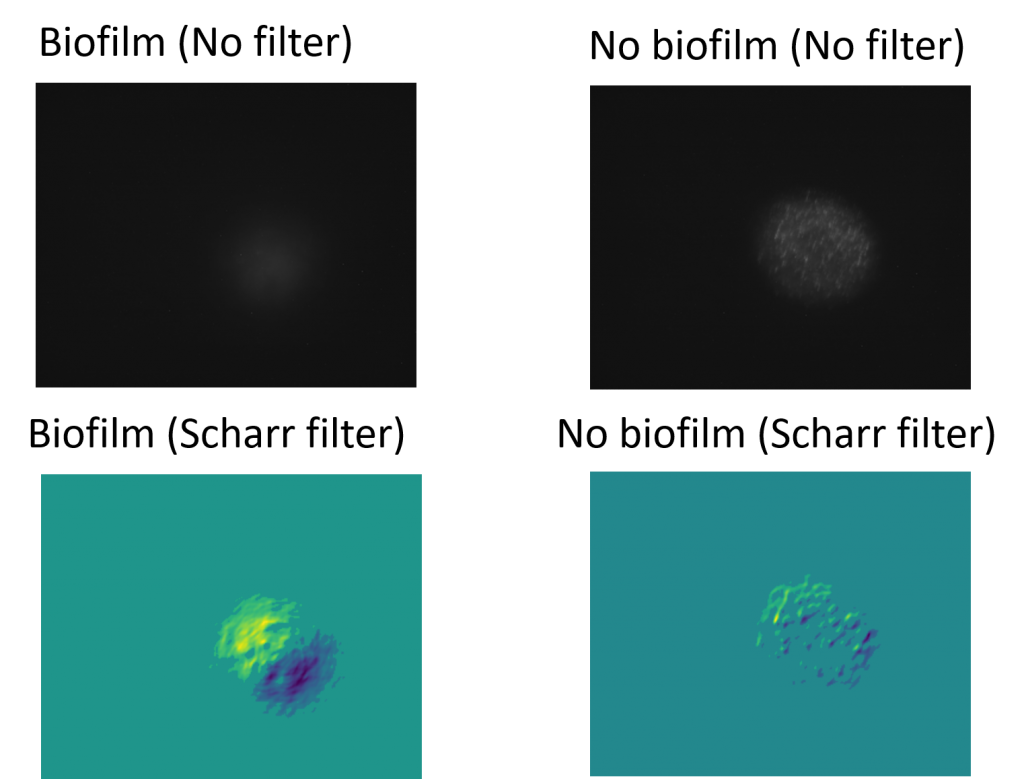
Alongside the two-dimensional processed images, a one-dimensional lineout which looks at the magnitudes from a single row or column also provide a distinction in the images. The lineout of the filter on the biofilm has a distinct peak with a rise and drop beforehand, indicating an edge. This is contrasted with the lineout of the filtered image without a biofilm which has no singular peak or trough.
Conclusions
Based on the analysis from the data, it can be concluded that imaging of biofilms compared to the base substrate produces substantial differences after post-processing. These differences can be used as a heuristic to determine a likelihood of a biofilm being present, or to determine the boundaries of a present biofilm. If an a priori background image is known, then the presence of a biofilm can be determined by analyzing the contrast of it and a current image of unknown property.
Further Plans
Due to unforeseen circumstances regarding lab closures and curriculum changes, not all data could be collected, and venues of inquiry explored. However, future expectations for the discoveries made in this project include infrared imaging of bone to detect fractures and to image materials with biofilm on the back side instead of the front side. Alongside this, refinement of image processing software and collection of data with different independent variables such as laser frequency, power, distance, etc. will continue. If future results show promise, a more direct focus on biomedical applications from biofilm imaging will also be explored.
Acknowledgements
I would like to thank Boise State University, Idaho INBRE and the Idaho Stem Action Center for allowing me this opportunity. I would also like to thank my mentor, Nirmala Kandadai, alongside my fellow lab mates James Lamb, Marcus Pearlman, and Attila Rektor. They all contributed large amounts to making this project successful and encouraging my personal growth and progress.
Additional Information
For questions or comments about this research, contact Noah Guidara at noahguidara@u.boisestate.edu.