Miranda Fischer, Brandi Sweet, Dr. Stephanie Hall, Dr. Luke Montrose, Erica Rowe
Abstract
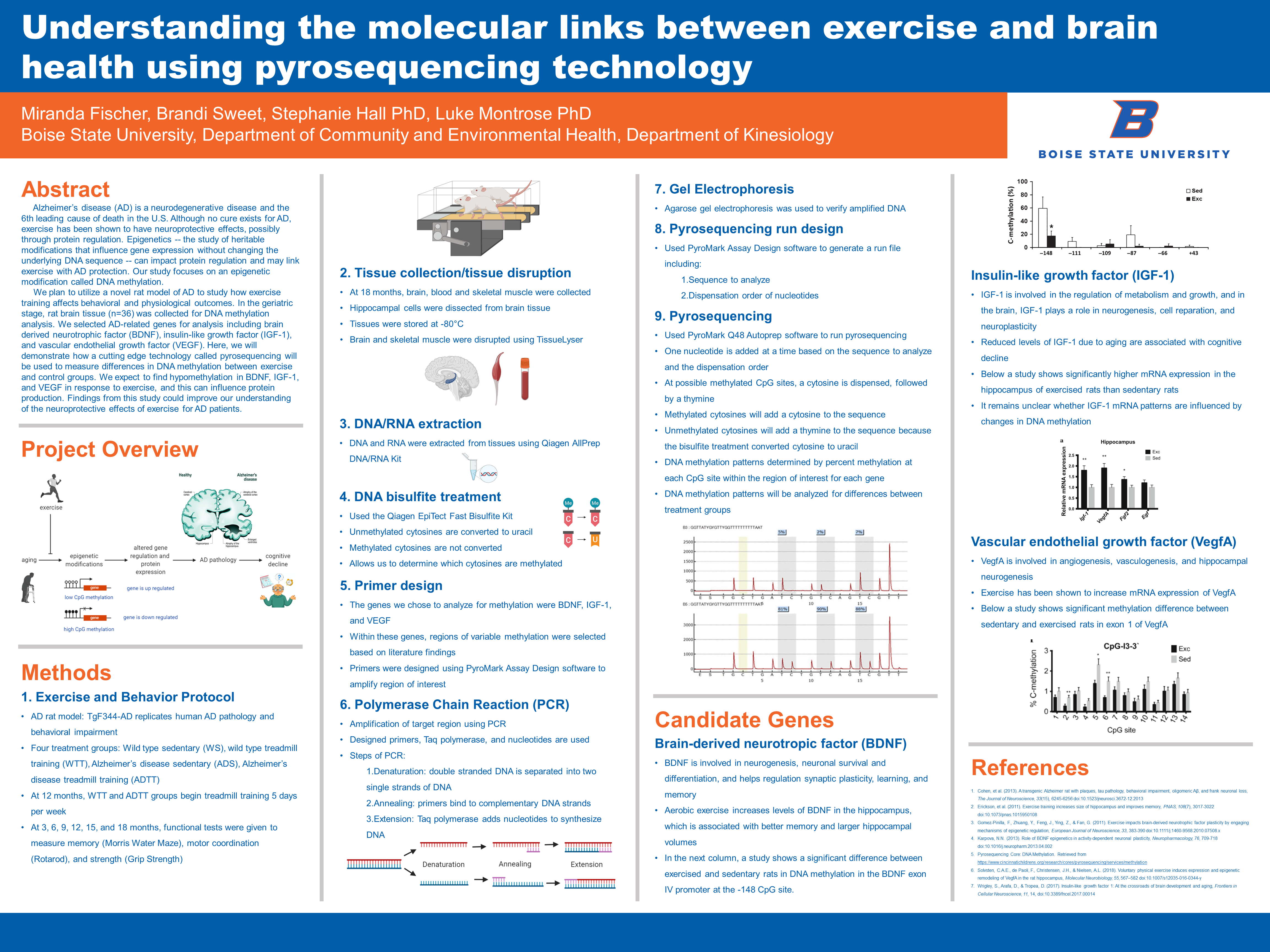
Alzheimer’s disease (AD) is a neurodegenerative disease and the 6th leading cause of death in the U.S. Although no cure exists for AD, exercise has been shown to have neuroprotective effects, possibly through protein regulation. Epigenetics — the study of heritable modifications that influence gene expression without changing the underlying DNA sequence — can impact protein regulation and may link exercise with AD protection. Our study focuses on an epigenetic modification called DNA methylation.
We plan to utilize a novel rat model of AD to study how exercise training affects behavioral and physiological outcomes. In the geriatric stage, rat brain tissue (n=36) was collected for DNA methylation analysis. We selected AD-related genes for analysis including brain derived neurotrophic factor (BDNF), insulin-like growth factor (IGF-1), and vascular endothelial growth factor (VEGF). Here, we will demonstrate how a cutting edge technology called pyrosequencing will be used to measure differences in DNA methylation between exercise and control groups. We expect to find hypomethylation in BDNF, IGF-1, and VEGF in response to exercise, and this can influence protein production. Findings from this study could improve our understanding of the neuroprotective effects of exercise for AD patients.
Project Overview
Methods
1. Exercise and Behavior Protocol
- AD rat model: TgF344-AD replicates human AD pathology and behavioral impairment
- Four treatment groups: Wild type sedentary (WS), wild type treadmill training (WTT), Alzheimer’s disease sedentary (ADS), Alzheimer’s disease treadmill training (ADTT)
- At 12 months, WTT and ADTT groups begin treadmill training 5 days per week
- At 3, 6, 9, 12, 15, and 18 months, functional tests were given to measure memory (Morris Water Maze), motor coordination (Rotarod), and strength (Grip Strength)
2. Tissue collection/tissue disruption
- At 18 months, brain, blood and skeletal muscle were collected
- Hippocampal cells were dissected from brain tissue
- Tissues were stored at -80°C
- Brain and skeletal muscle were disrupted using TissueLyser
3. DNA/RNA extraction
-
- DNA and RNA were extracted from tissues using Qiagen AllPrep DNA/RNA Kit
4. DNA bisulfite treatment
- Used the Qiagen EpiTect Fast Bisulfite Kit
- Unmethylated cytosines are converted to uracil
- Methylated cytosines are not converted
- Allows us to determine which cytosines are methylated
5. Primer design
- The genes we chose to analyze for methylation were BDNF, IGF-1, and VEGF
- Within these genes, regions of variable methylation were selected based on literature findings
- Primers were designed using PyroMark Assay Design software to amplify region of interest
6. Polymerase Chain Reaction (PCR)
- Amplification of target region using PCR
- Designed primers, Taq polymerase, and nucleotides are used
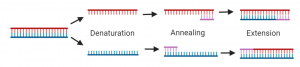 Steps of PCR:
Steps of PCR:
- Denaturation: double stranded DNA is separated into two single strands of DNA
- Annealing: primers bind to complementary DNA strands
- Extension: Taq polymerase adds nucleotides to synthesize DNA
7. Gel Electrophoresis
- Agarose gel electrophoresis was used to verify amplified DNA
8. Pyrosequencing run design
- Used PyroMark Assay Design software to generate a run file including:
- Sequence to analyze
- Dispensation order of nucleotides
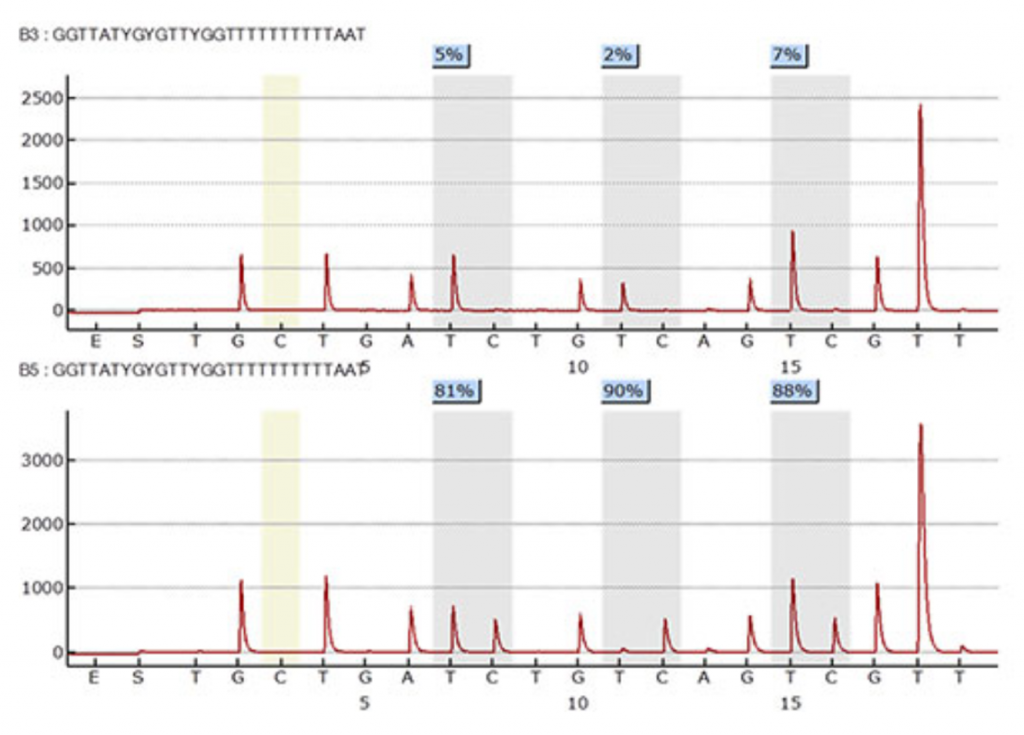
9. Pyrosequencing
- Used PyroMark Q48 Autoprep software to run pyrosequencing
- One nucleotide is added at a time based on the sequence to analyze and the dispensation order
- At possible methylated CpG sites, a cytosine is dispensed, followed by a thymine
- Methylated cytosines will add a cytosine to the sequence
Unmethylated cytosines will add a thymine to the sequence because the bisulfite treatment converted cytosine to uracil - DNA methylation patterns determined by percent methylation at each CpG site within the region of interest for each gene
- DNA methylation patterns will be analyzed for differences between treatment groups
Candidate Genes
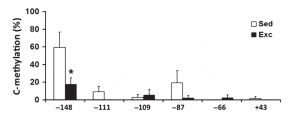
Brain-derived neurotropic factor (BDNF)
- BDNF is involved in neurogenesis, neuronal survival and differentiation, and helps regulation synaptic plasticity, learning, and memory
- Aerobic exercise increases levels of BDNF in the hippocampus, which is associated with better memory and larger hippocampal volumes
- In the next column, a study shows a significant difference between exercised and sedentary rats in DNA methylation in the BDNF exon IV promoter at the -148 CpG site.
Insulin-like growth factor (IGF-1)
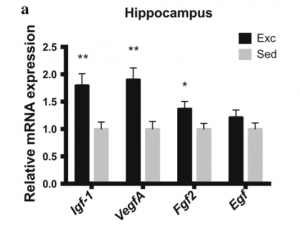 IGF-1 is involved in the regulation of metabolism and growth, and in the brain, IGF-1 plays a role in neurogenesis, cell reparation, and neuroplasticity
IGF-1 is involved in the regulation of metabolism and growth, and in the brain, IGF-1 plays a role in neurogenesis, cell reparation, and neuroplasticity- Reduced levels of IGF-1 due to aging are associated with cognitive decline
- Below a study shows significantly higher mRNA expression in the hippocampus of exercised rats than sedentary rats
- It remains unclear whether IGF-1 mRNA patterns are influenced by changes in DNA methylation
Vascular endothelial growth factor (VegfA)
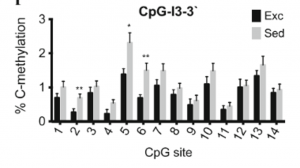 VegfA is involved in angiogenesis, vasculogenesis, and hippocampal neurogenesis
VegfA is involved in angiogenesis, vasculogenesis, and hippocampal neurogenesis- Exercise has been shown to increase mRNA expression of VegfA
- Below a study shows significant methylation difference between sedentary and exercised rats in exon 1 of VegfA
References
- Cohen, et al. (2013). A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss, The Journal of Neuroscience, 33(15), 6245-6256 doi:10.1523/jneurosci.3672-12.2013
- Erickson, et al. (2011). Exercise training increases size of hippocampus and improves memory, PNAS, 108(7), 3017-3022 doi:10.1073/pnas.1015950108
- Gomez-Pinilla, F., Zhuang, Y., Feng, J., Ying, Z., & Fan, G. (2011). Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation, European Journal of Neuroscience, 33, 383-390 doi:10.1111/j.1460-9568.2010.07508.x
- Karpova, N.N. (2013). Role of BDNF epigenetics in activity-dependent neuronal plasticity, Neuropharmacology, 76, 709-718 doi:10.1016/j.neuropharm.2013.04.002
- Pyrosequencing Core: DNA Methylation. Retrieved from https://www.cincinnatichildrens.org/research/cores/pyrosequencing/services/methylation
- Solvsten, C.A.E., de Paoli, F., Christensen, J.H., & Nielsen, A.L. (2018). Voluntary physical exercise induces expression and epigenetic remodeling of VegfA in the rat hippocampus, Molecular Neurobiology, 55, 567–582 doi:10.1007/s12035-016-0344-y
- Wrigley, S., Arafa, D., & Tropea, D. (2017). Insulin-like growth factor 1: At the crossroads of brain development and aging, Frontiers in Cellular Neuroscience, 11, 14, doi:10.3389/fncel.2017.00014
Additional Information
For questions or comments about this research, contact Miranda Fischer at mirandafischer@u.boisestate.edu.