Flow Cytometry Core Facility

- About the Facility
- Faculty/Staff, Contact Information
- Instrument Capabilities – Sony SH800S & BD Influx Cell Sorter
- Cell Sorting Rates & Specs
- Applications
- Understanding Flow Cytometry
- Additional Resources
About the Facility
The Flow Cytometry Core Facility (FCCF) at Boise State University was created to aid researchers and graduate students in implementing flow cytometry into their experimentation. The facility’s services, housed in the Science building (Room 218), are available primarily to the Boise State University and Treasure Valley communities. The FCCF has a Sony SH800S cell sorter with three lasers and six detectors (listed below). This instrument is capable of two-way sorting into various tube volumes, as well as sorting into plates. The SH800S is highly automated and features automatic compensation to account for spillover between detectors. The instrument is microfluidic based using a chip to create droplets encapsulating a sample. There are three different chip sizes to account for various size cells or particles.
The FCCF is also equipped with a state of the art four-laser BD INFLUX cell sorter. This instrument supports four-way sorting, plate sorting for single cell isolation, 9-color analysis, and can operate at up to 200,000 events per second. The BD INFLUX at BSU is also equipped with a small particle detector allowing particle detection near 200 nm.


Faculty/Staff, Contact Information
To schedule time or consultation for services, please contact Dr. Covert.
- Dr. Denise Wingett – Director, Flow Cytometry Core Facility; Professor, Department of Biological Sciences; Director, Biomolecular Sciences Ph.D. Program; (208) 426-2921; Denisewingett@boisestate.edu
- Dr. Hunter Covert – Staff, Flow Cytometry Core Facility. Department of Biological Sciences, Department of Biomolecular Sciences. (208) 426-5940; huntercovert@boisestate.edu
Rates & Specs
Sony SH800S cell sorting and flow cytometry rates are offered to internal users at $100/hour, and $142.50/hour for external users. Please inquire within for Influx hourly rates. User is asked to provide their own vessel containing the sample and vessel for collection of desired cells.
Technology Access Grants are available through INBRE Idaho. Apply here: https://inbre.uidaho.edu/inbre-bioinformatics-core-technology-access-awards/
Eligibility:
- Idaho biomedical researchers with an appointment at one of the Idaho INBRE partner institutions (BSU, BYU-I, COI, CSI, CWI, ISU, IVREF, LCSC, NIC, NNU, UI).
- Postdoctoral fellows and graduate students of eligible PI’s are also eligible to apply, but must include a brief letter of support from the PI/mentor.
- Projects must reflect overall biomedical research goals of the INBRE program.
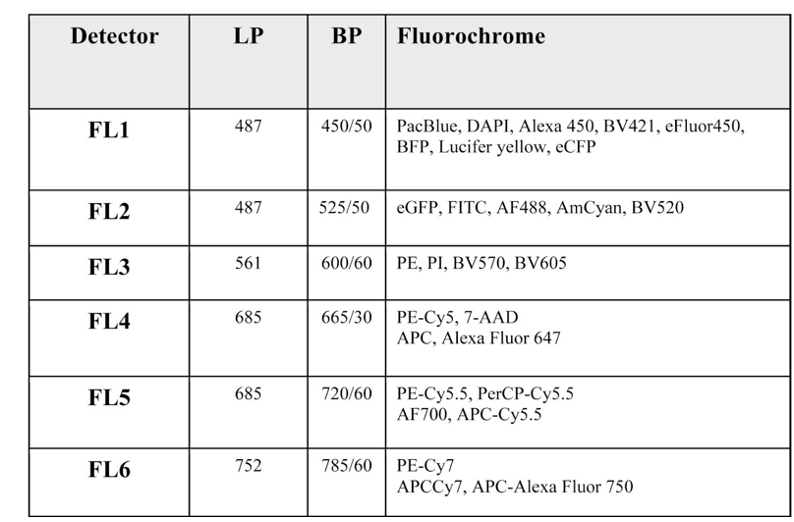
Instrument Specs
Sony SH800S:
Lasers: 488nm, 561nm and 638nm
Detectors:
Applications
There are many uses for flow cytometry, many of which are outlined in this video by Beckman Coulter. Examining, counting, and sorting cells are the primary functions of this technology. Samples are sorted primarily based on the presence of a fluorescent dye, but also size and granularity characteristics. These characteristics assist researchers in the following examples of applications of flow cytometry:
- Analyze target (Ab bound, fluorescent) versus non-target cells
- Measure DNA content of a cell sample
- Cell cycle analysis determination
- RNA vs DNA differentiation during cell cycle progression
- Distinguish between immature and mature RBC
- Evaluate cell viability
- Investigate cytokine production from activated cells
- Monitor second messengers to determine cell activation state
Understanding Flow Cytometry
Flow cytometry analyzes cells based on relative size, granularity, and fluorescence. A flow cytometer is composed of three main systems: fluidics, optics, and electronics. The fluidics system operates to inject the cell sample into the sheath fluid for delivery to the laser for interrogation. Hydrodynamic focusing of the sample within the sheath fluid allows for one cell to pass through the laser at a time. The optical system contains lasers, lenses, and filters. As a cell passes through the laser beam it will scatter light for relative size and granularity measurements. Lasers also excite fluorochromes present in the cell sample. Lenses and filters are required to direct the scattered and excited light of specific wavelengths to various detectors for collection. The electronics system converts the light signals received by the detectors to electronic signals using photodetectors (photodiodes and photomultiplier tubes). These converted light signals are displayed on a graph, such as a dot plot or histogram, that can be used to analyze, gate, or sort a selected cell population in the sample.
Additional Resources
Additional resources regarding flow cytometry can be found at the following links:
- For help selecting antibodies and designing a flow cytometry panel, visit FluoroFinder
- Beckman Coulter’s Computer Based Training: Animated Flow Cytometry Theory
- BD Bioscience Introduction to Flow Cytometry: A Learning Guide (PDF)
- BD Bioscience BD Flow Cytometry: Technical Bulletin
- Thermo Fisher Scientific Flow Cytometry Learning Center