More so than ever before, research today is of necessity a collaborative undertaking. As knowledge has expanded, specialization has occurred. As a result, science and engineering disciplines have proliferated. Accompanying these developments is also the realization that the underlying science and engineering necessary to address societal needs, challenges, and aspirations have become multifaceted and complex, and that the work identified through societal input often cannot be addressed by single investigator, single discipline, or even single institution approaches.
One such example of a complex and societally relevant challenge is the arena of quantum information science (QIS). The basis of QIS delves into the underpinnings of quantum physics. And its applications, or quantum information technology, potentially impact a broad swath of economic and societal interests, including quantum computing (faster computers to solve currently unsolvable problems in medicine, materials research, weather forecasting, space travel, etc.), quantum sensing (highly sensitive sensors), entangled photon emitters (entangled light source to examine emergent light-matter interactions), and quantum communication (transfer of information with high fidelity and protection) to name a few. The importance and impact of QIS has captured the attention of nations worldwide. As one example, on December 21, 2018 the National Quantum Initiative Act was signed into law, which supports quantum research and development in the United States. To understand QIS, and successfully apply that understanding to address societal needs, will require not only insights from multiple disciplines, but also new types of collaborations among industry, government laboratories, and academic institutions.
The Quantum DNA (qDNA) Research Group, which consists of a team of scientists and engineers in the Nanoscale Materials and Device Group in the Micron School of Materials Science and Engineering at Boise State University, is actively engaged in QIS research via a number of federal research grants. This article highlights three of the group’s recent activities (i.e., journal publications) in which the three types of collaborations mentioned above are involved. Such collaborations are key to (1) advancing science and engineering from a transdisciplinary perspective, (2) fostering partnerships that connect and leverage fundamental science and engineering to drive and accelerate applications essential to meeting societal needs, (3) exposing Boise State students and early career research staff to a broader community of academic, industrial, and governmental interests with whom their work has relevance, and (4) enhancing Boise State’s capacity to compete at the national and international levels to the economic benefit of Idaho and the United States as a whole.
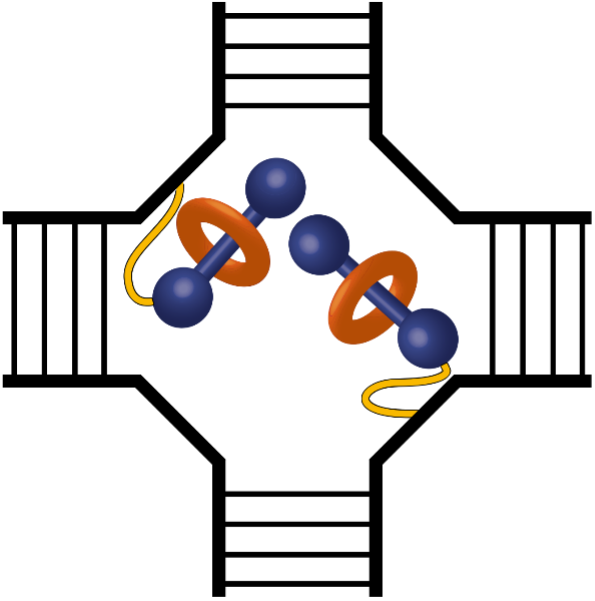
The first example is an article published in the journal Communications Chemistry by Dr. Matthew Barclay, a Postdoctoral Research Fellow in the qDNA group and first author of the manuscript. Matt and his co-authors highlight how DNA (i.e., deoxyribonucleic acid) can be used to assemble aggregates of novel dye molecules. Because of their strong ability to absorb light, dyes (or pigments) are used to color clothes and paints. As a result, they also can be used to design and develop a material with optical properties that may be useful for QIS. In particular, Matt and co-workers showed that bringing together two dye molecules, which had not intentionally been brought together before, promotes a hard to achieve packing arrangement and certain type of color change that may benefit fundamental studies in QIS, such as quantum entanglement. For a material to take on such a color change, the dyes within the material need to be within one to two nanometers of each other. To accomplish this close dye placement, nature is the inspiration. Plants, algae, and certain bacteria, for example, use proteins to arrange dyes (e.g., chlorophyll) with nanometer-scale precision. Protein building blocks, specifically amino acids, number over 20. Thus, the design rules to create protein-templated dye aggregates are complicated. Inspired by nature, but looking for a simpler solution, Matt and colleagues relied on the self-assembly behavior of DNA. With only four nucleic acid building blocks, the design rules of DNA self-assembly are significantly less complex. Thus, a DNA scaffold was used to arrange the dye molecules to within a nanometer of one another. One of the most novel aspects of the work was the assembly of the dyes themselves in the form of a rotaxane composite molecule: that is, a molecule composed of a macrocycle (or ring) that encapsulates a dye to form a rotaxane (as highlighted by the 2016 Nobel Prize in Chemistry). Macrocycles can provide a protective environment, or cage, around the dye; for example, by hindering access to a chemically reactive part of the molecule with an outer ring that is non-reactive. In the study by Matt and his co-authors, the rotaxane ring surrounds a squaraine dye and enables these typically non-aggregating dyes to assume an oblique (mostly orthogonal) packing arrangement with resultant optical properties that may benefit QIS. The dyes themselves come out of a unique relationship with an industry partner, SETA BioMedicals, who supply dyes, such as fluorescent detection reagents, for biomedical applications. The industrial relationship enables Boise State researchers to customize the dye design process—necessary to understand and control dye structure-property relationships—and then to procure these dyes from SETA BioMedicals.
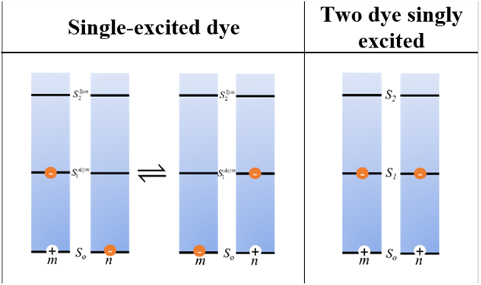
The Quantum DNA Research Group has a rich history of collaboration with the U.S. Naval Research Laboratory (NRL), which constitutes our second example. Since 2016, the NRL collaboration has enriched the advancement and development of novel research ideas and enhanced professional development for Boise State students and graduates. For example, NRL has leveraged Boise State’s considerable experience with ultrafast laser spectroscopy, whereas NRL’s experience and expertise with dye synthesis and cryogenic (77 K or -321 oF) spectroscopy has catalyzed Boise State’s initiation into acquiring cryogenic spectroscopy capability. NRL scientists have served on dissertation committees of Boise State graduate students and hired three Boise State graduates as post-doctoral researchers. In a recent collaboration resulting in a publication in the Journal of Physical Chemistry B, Boise State’s Distinguished Research Fellow, Dr. Bernard Yurke, contributed the theoretical quantum insights underlying experimental results obtained by NRL. Quantum theory predicts that dye networks organized via DNA self-assembly into a DNA-dye aggregate scaffold have the potential to serve as platforms for molecular quantum logic gates (for quantum computing purposes) and entangled photon emission based on delocalized two-exciton states. An exciton is an electrically neutral quasiparticle consisting of an electron (negatively charged) and hole (positively charged). An exciton is created when a dye molecule in an aggregate of dyes is excited by and captures light. This exciton is shared between dyes or delocalized across the dyes: that is, the exciton’s position at any time is likely to be as much on one dye as the other. This phenomenon is known as superposition, in which the exciton is shared by the two dyes simultaneously. It is a simple example of spatial quantum entanglement as the exciton is “entangled” between the two dyes. In the research captured here, however, NRL-Boise State researchers demonstrated that templating cyanine dyes into a DNA-dye aggregate scaffold in a closely spaced manner also can be a controllable method for creating two-exciton states, in which an exciton exists on each dye and thus interact with one another.
Our third example demonstrates a highly productive collaboration across academic institutions, in which Boise State’s Senior Research Scholar, Dr. Daniel Turner, and his colleague from California State University at Chico, Dr. Paul Arpin, have followed their recently published article (see Boise State Update August 25, 2020) with a new contribution. This time, in an article published in the Journal of Physical Chemistry A, they have tackled expanding the theoretical basis for how to identify electronic versus vibrational signatures, or quantum beats, that can arise in measurements of molecular aggregates. Quantum beats arise from the superposition of an exciton between two energy levels (excited states) whose detectable optical signals are oscillatory (similar to beat frequency in music). The duration of the oscillations provides an indication of the time the system remains coherent (i.e., the wavelength and phase of the oscillations remain unchanged). Quantum computing requires a system to be coherent. Once the system loses coherence, quantum computation is no longer achievable. The duration of quantum beats can be impacted by heat. Even at room temperature, the dyes absorb heat from their surroundings. This causes the atoms in the dyes to vibrate like weights attached to a spring. When an exciton is created in such a dye molecule, the vibration essentially jostles the exciton. The result is that quantum beats are suppressed by the vibrations of the dye and the system loses coherence. It makes sense then that an exciton’s lifetime is shortened in the presence of vibrations. The work of Drs. Arpin and Turner provides another tool in the toolbox to distinguish between electronic and vibrational signatures and thereby also identify possible mechanisms by which to enhance electronic coherence.
Citations, links to, and funding sources of the publications discussed in the article
S. Barclay, S. K. Roy*, J. S. Huff*, O. A. Mass, D. B. Turner, C. K. Wilson, D. L. Kellis, E. A. Terpetschnig, J. Lee, P. H. Davis, B. Yurke, W. B. Knowlton and R. D. Pensack, Rotaxane Rings Promote Oblique Packing and Extended Lifetimes in DNA-Templated Molecular Dye Aggregates, Communications Chemistry, 4 (19), 1–11 (2021). doi.org/10.1038/s42004–021–00456–8.
*Denotes a graduate student. Research at Boise State University, including data collection, analysis, and interpretation and manuscript preparation, was supported by the U.S. Department of Energy (DOE), Office of Basic Energy Sciences, Division of Materials Science and Engineering through the Established Program to Stimulate Competitive Research (EPSCoR) via award No. DE-SC0020089. Specific equipment was supported by additional funding sources (see link to paper above for full funding source acknowledgments).
D. Cunningham, S. A. Díaz, B. Yurke, I. L. Medintz, and J. S. Melinger, Delocalized Two-Exciton States in DNA Scaffolded Cyanine Dimers, Journal of Physical Chemistry B, 124(37), 8042–8049 (2020). doi.org/10.1021/acs.jpcb.0c06732.
Dr. Yurke’s research was supported by the National Science Foundation INSPIRE (Integrated NSF Support Promoting Interdisciplinary Research and Education) program under award number 1648655.
C. Arpin and D. B. Turner, Signatures of Vibrational and Electronic Quantum Beats in Femtosecond Coherence Spectra, Journal of Physical Chemistry A, 125(12), 2425–2435 (2021). doi.org/10.1021/acs.jpca.0c10807.
Dr Turner’s research was supported by the Department of the Navy, Office of Naval Research (ONR) under ONR award number N00014–19–1–2615.
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the DOE’s Office of Basic Energy Sciences, Materials Sciences and Engineering Division, DOE’s EPSCoR program, DOE’s Idaho National Laboratory, Department of the Navy’s ONR, or the National Science Foundation.