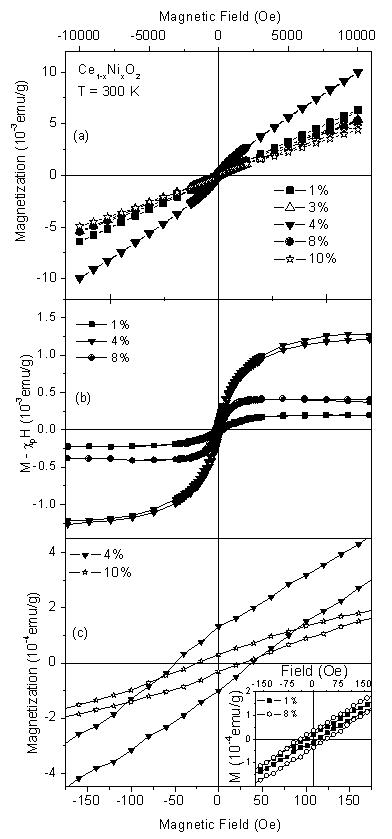
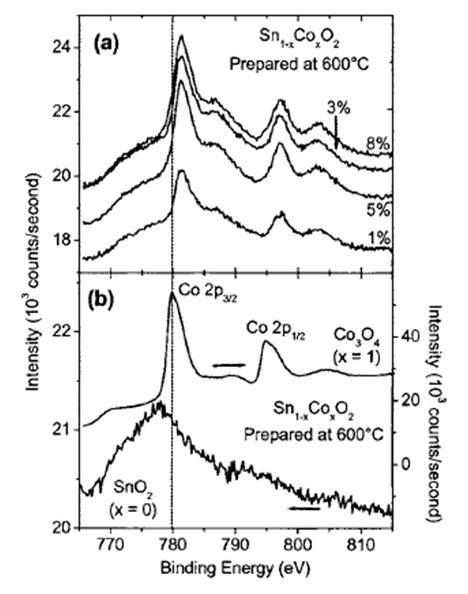
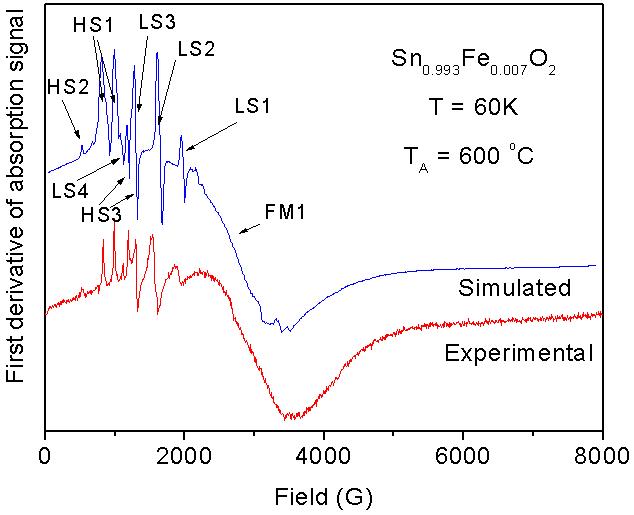
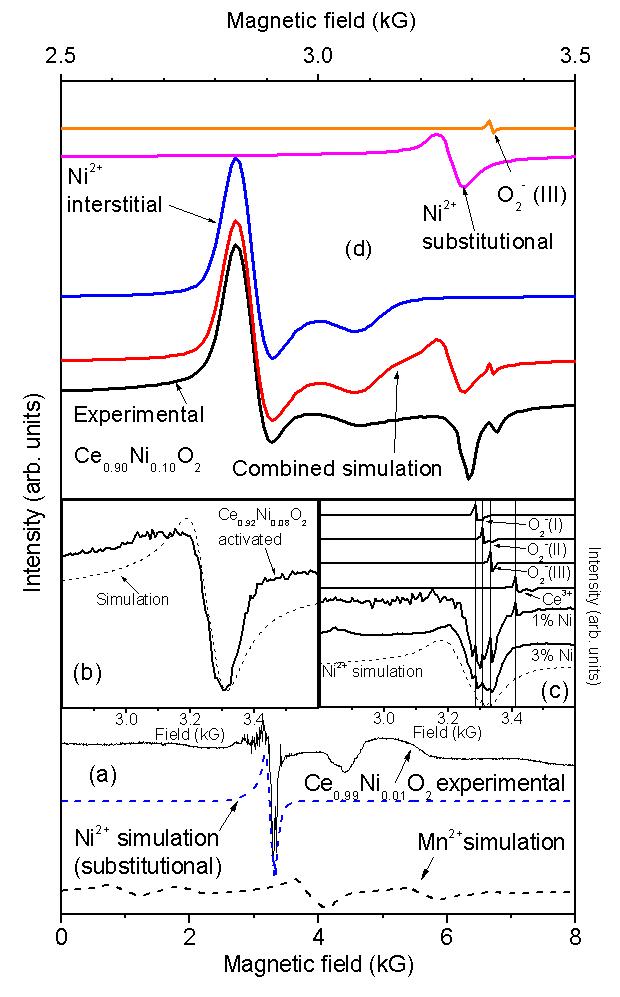
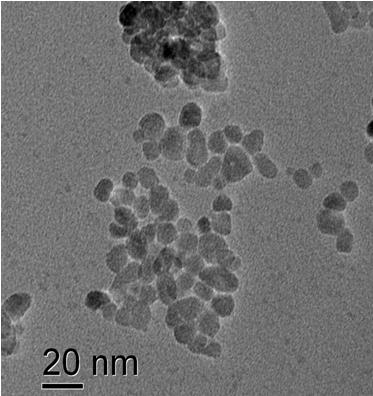
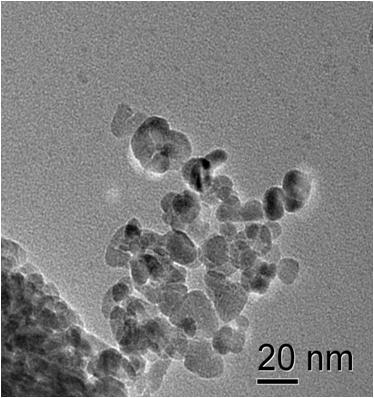
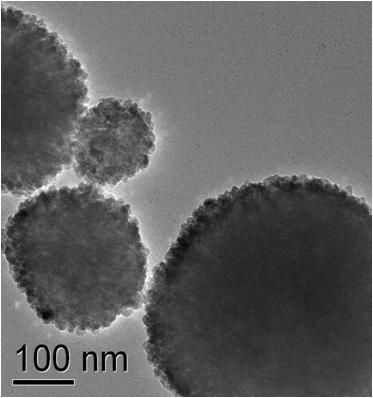
Electrical properties
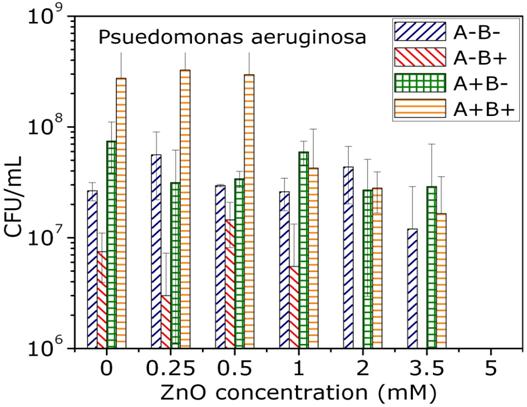
Toxicology studies
The toxicity of nanoparticles is determined for various bacterial organisms, cancer cells and healthy cells. ZnO nanoparticles show selective toxicity to the cancer cells. The TEM image shows ZnO nanoparticles showing affinity towards attaching onto a bacteria cell. Nanoparticles of other oxides are under investigation to study these effects.