Dalton Miller, Jasper Theel, Adam Croteau, Brett Nelson, Dr. Jim Browning, Dr. Ken Cornell

Abstract
The CDC estimates that 128,000 people are hospitalized due to foodborne illness each year in the United States. The presence of bacterial biofilms in food-processing settings is a concern for the spread of disease, and is responsible for a significant number of the outbreaks that result in hospitalizations and food recalls. Although food-processing plants can be sterilized to some degree, the current means of doing so uses harsh chemicals and requires production to be halted for extended periods of time. To that end, we have developed a novel cold atmospheric-pressure plasma (CAPP) device to combat these types of biofilms in a more cost effective manner that requires no harsh chemicals. This biofilm removal can be imaged using fluorescence microscopy and quantified by profilometry, which measures the height of the biofilm before and after CAPP treatment. Here we demonstrate that even short (e.g. 5 minutes) CAPP treatments could etch away biofilms in a time-dose dependent fashion. Our findings provide a proof-of-concept that a CAPP device is a viable potential alternative to classic food processing decontamination methods that rely on harsh chemicals.
Hypothesis
CAP plasma device can be used to deliver ionized gas to kill bacterial biofilms on multiple substrates including industrial surfaces and organic models.
Materials and Methods
Plasma Experiments:
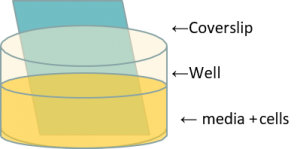 2 day biofilms incubation at 37°C in 12 well plates
2 day biofilms incubation at 37°C in 12 well plates- Rinse with PBS
- Pseudomonas fluorescens used.
CAP Device:
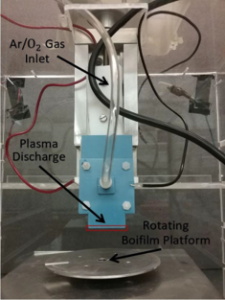
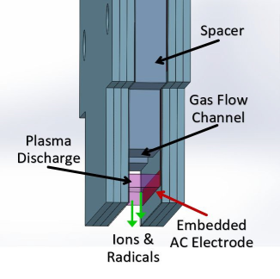 Plasma is generated due to the parallel plate capacitive discharge that occurs between the
Plasma is generated due to the parallel plate capacitive discharge that occurs between the
low-temperature co-fired ceramic (LTCC) plates.- Parameters:
- Exposure distance: 3 mm
- Exposure time: 5 min
- Source voltage: 2.1 kV
- Source frequency: 20 kHz
- Type: Wide 1”
- Argon flow: 9 slm
Profilometer:
 Bruker DekTak XT Stylus Profilometer Parameters:
Bruker DekTak XT Stylus Profilometer Parameters:
- 12.5 μm stylus
- Resolution: 0.25
- Can only use non-pathogenic bacteria
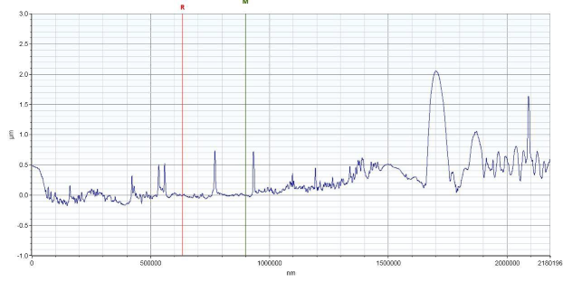
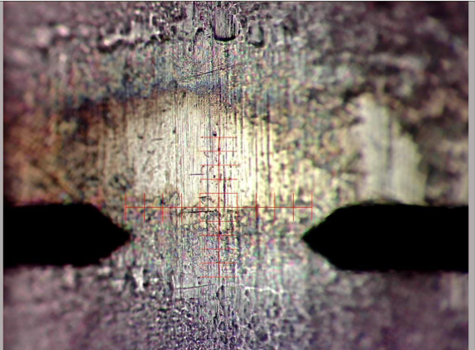
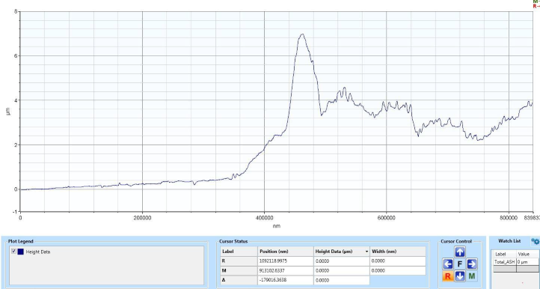
Results: Profilometry
Preliminary Conclusion: CAP treatment can remove bacterial biofilms from industrial substrate, and this removal can be measured via profilometry.
Future Work
Although etching was seen to be possible, it wasn’t consistent. Despite consistently using the same settings on the CAPP device, etching was not consistently seen. This is most likely due to an overlooked factor, but more testing is needed to determine what that factor is.
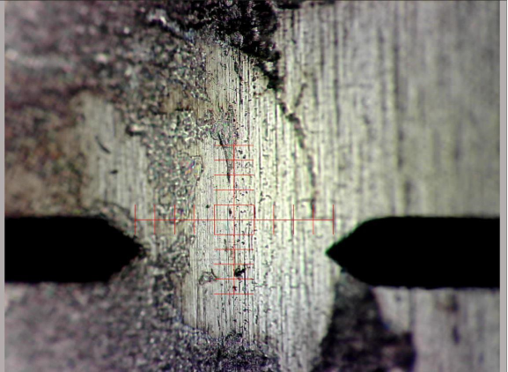
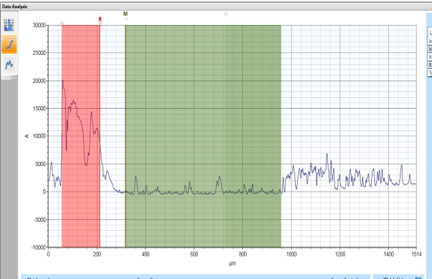
Optical Profilometry:
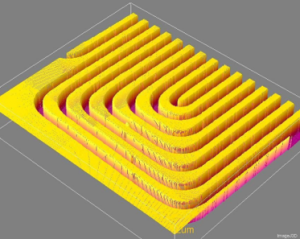
Optical profilometry could also be used in the same way, but would have some potential advantages, including:
- can be used with pathogenic bacteria, as it never makes contact with the surface
- Can produce 3-dimensional surface scans, as seen to the left in a sample scan of an industrial part
Acknowledgements
SG received support from the Ralph Jones Premedical Fellowship. This project was supported by Institutional Development Awards (IDeA) from the NIH NIGMS under Grants #P20GM103408 and #P20GM109095, the BSU Biomolecular Research Center, and the Vertically Integrated Projects program in the BSU College of Innovation and Design with funding from the Leona M. & Harry B. Helmsley Charitable Trust. Lastly, the project has received funding from the U.S. Dept. of Agriculture under Grant 2018-67018-27881.
Additional Information
For questions or comments about this research, contact Dalton Miller at daltonmiller@u.boisestate.edu.