Savannah Temple, Tim O’Donnell

If fastidious organisms are grown in their niche chemical, physical and nutritional conditions then they may exhibit prolonged stationary phase and increased sustainability
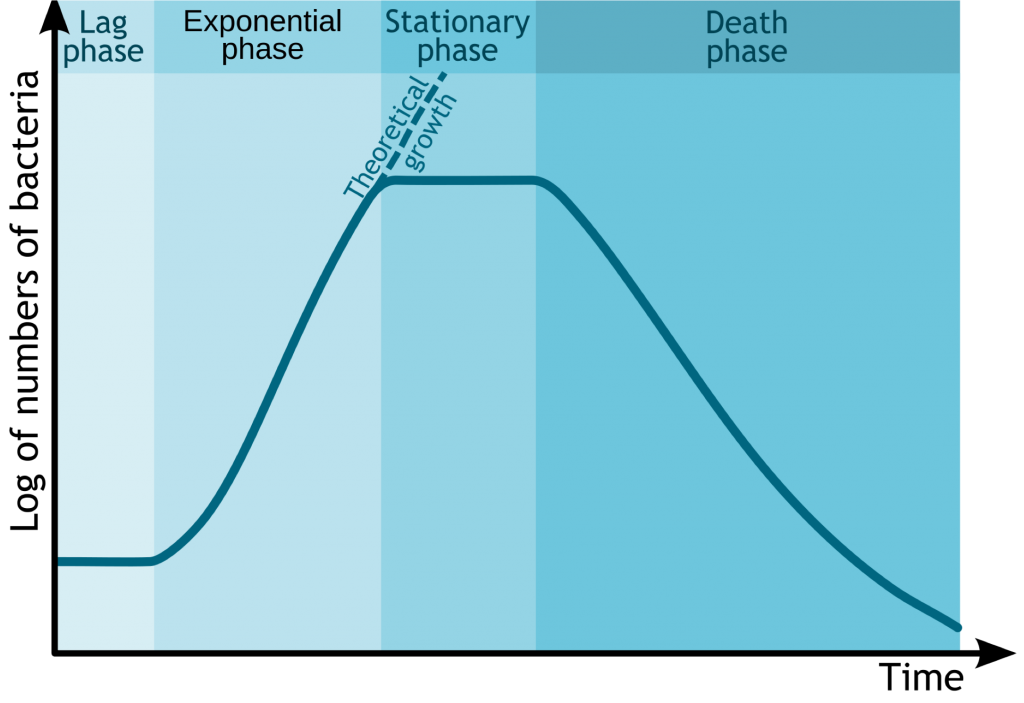
Background
Bacteria grow best at their optimum chemical, physical and nutritional conditions. Bacterial growth can be charted with a logarithmic growth curve depicting four phases:
- Lag Phase: Microorganism adapts itself to the growth conditions.
- Exponential Phase: Microorganisms doubles cell replication.
- Stationary Phase: Growth rate and death rate are equal due to growth limiting factors.
- Death Phase: Microorganism dies.
In order to increase a microorganisms sustainability you have to prevent the bacteria from killing itself – depleting the medias nutrition or producing inhibitory products. Fastidious organisms are even more difficult to maintain as they have very niche requirements that are hard to artificially replicate.
Goal
The goal of this research was to test varying media types and growth conditions to see if we could increase the ‘Stationary Phase’ – the sustainability – of three fastidious microorganisms: P. aeruginosa, L. lactis, and S. marcescens.
Methods
Treatments
- 26* C vs. 35* C
- TSB vs. NB vs. BHI vs. LB
- TSA vs. NA vs. BHIA vs. LA
- Shaken vs. Non-Shaken at 200 RPM
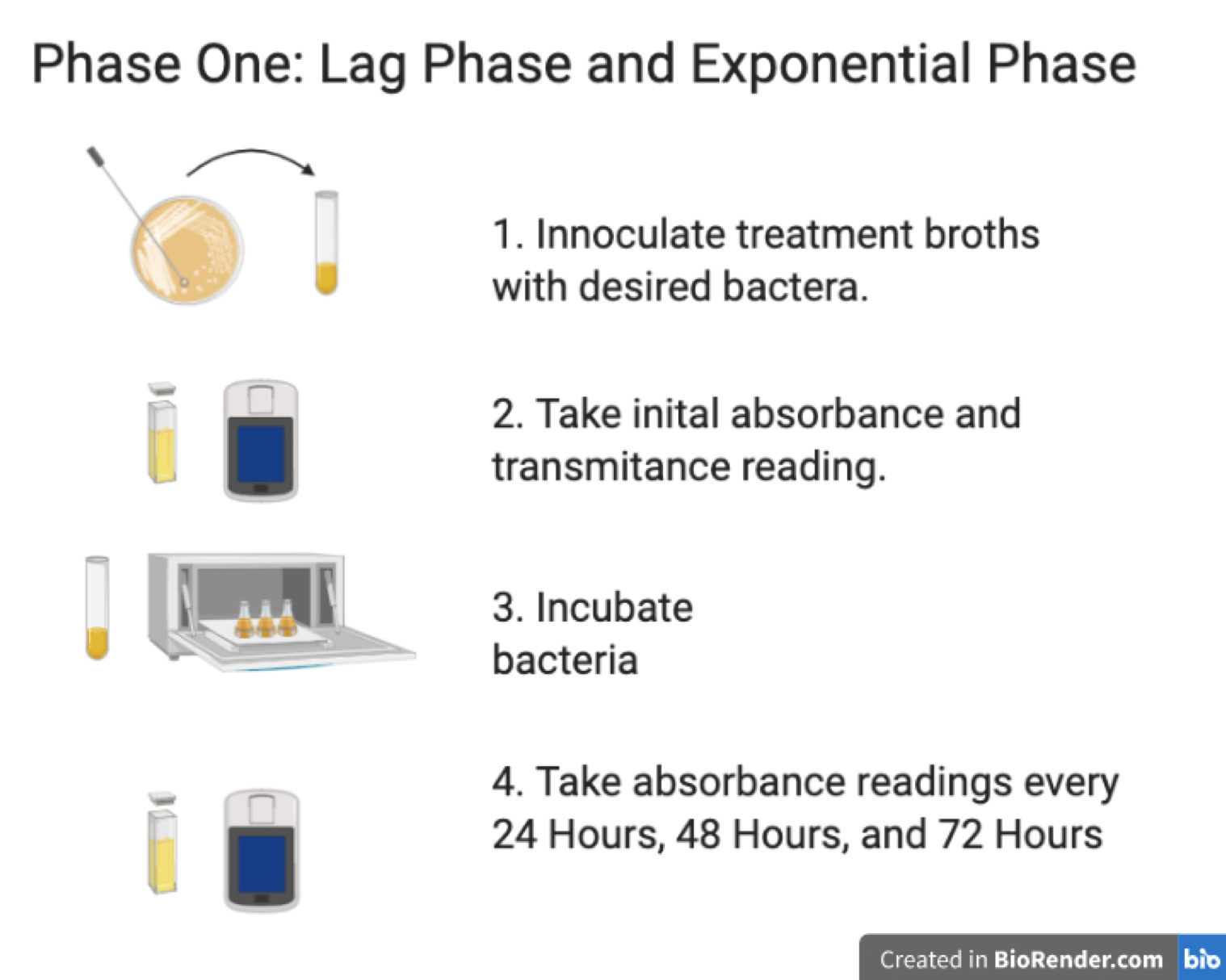
Phase One: Lag Phase and Exponential Phase
- Innoculate treatment broths with desired bacteria
- Take initial absorbance and transmitance reading
- Incubate bacteria
- Take absorbance readings every 24, 48, and 72 hours
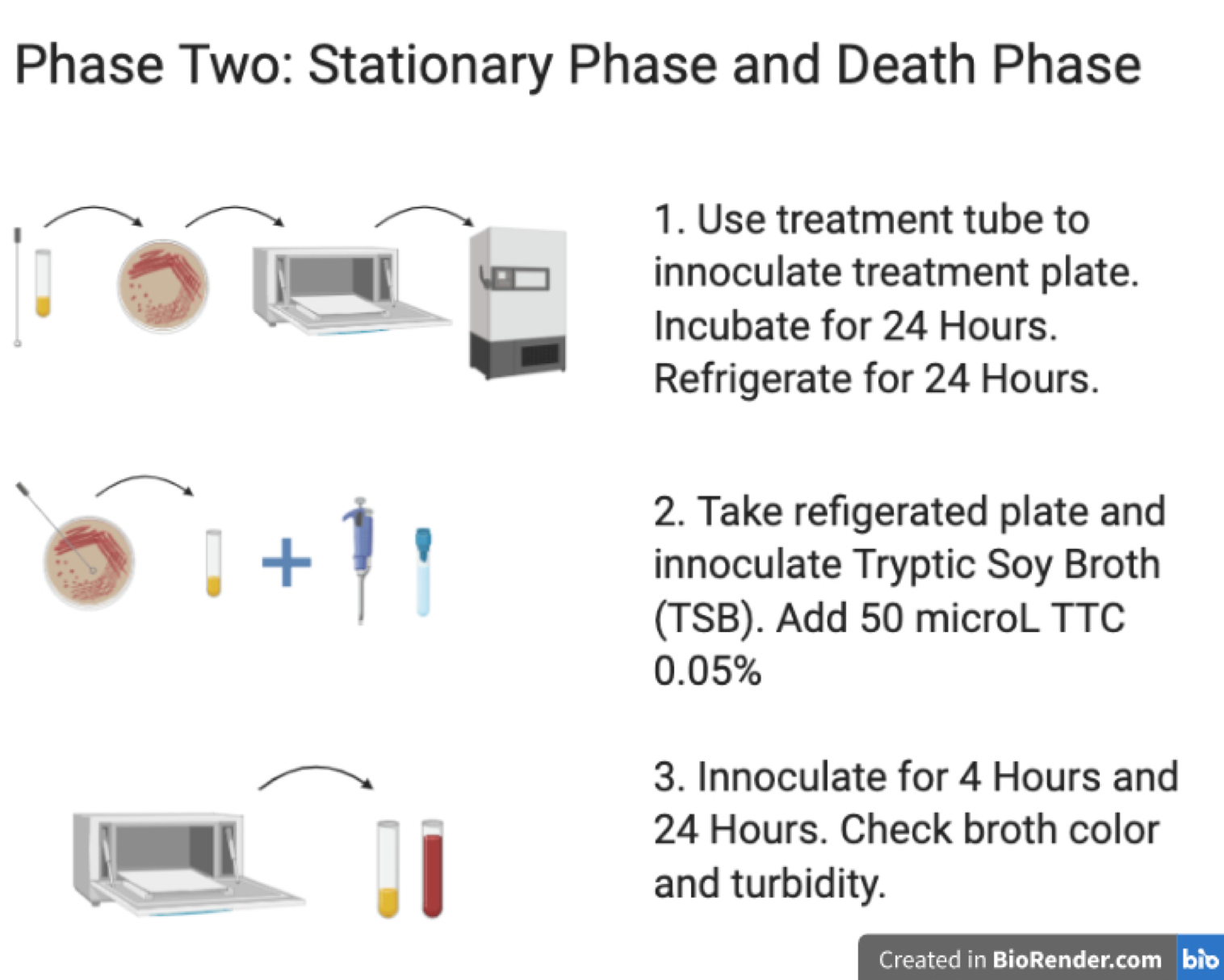
Phase two: Stationary Phase and Death Phase
- Use treatment tube to innoculate treatment plate. Incubate for 24 Hours. Refrigerate for 24 Hours.
- Take refrigerated plate and innoculate Tryptic Soy Broth (TSB). Add 50microL TTC 0.05%.
- Innoculate for 4 Hours and 24 Hours. Check broth color and turbidity.
Preliminary Data
Figure 2 Below: Four scatter plots depicting the relationship between the Absorbance level and Time in hours for four different treatment groups.
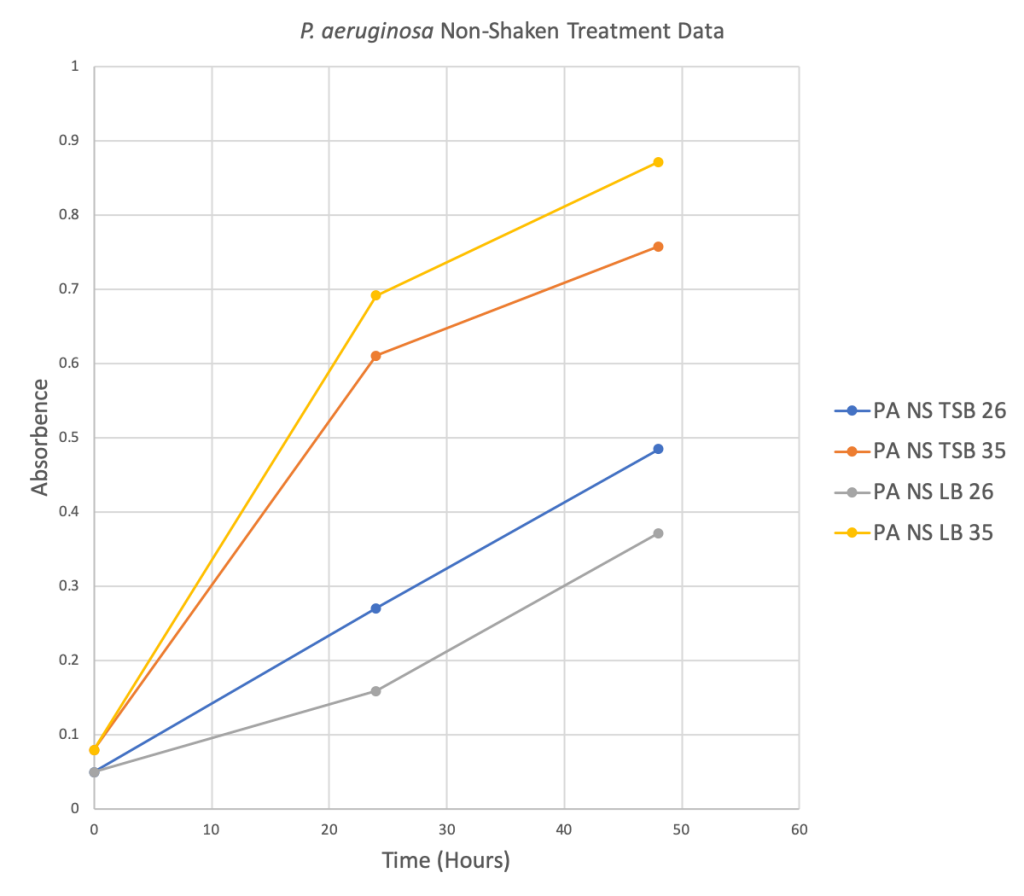
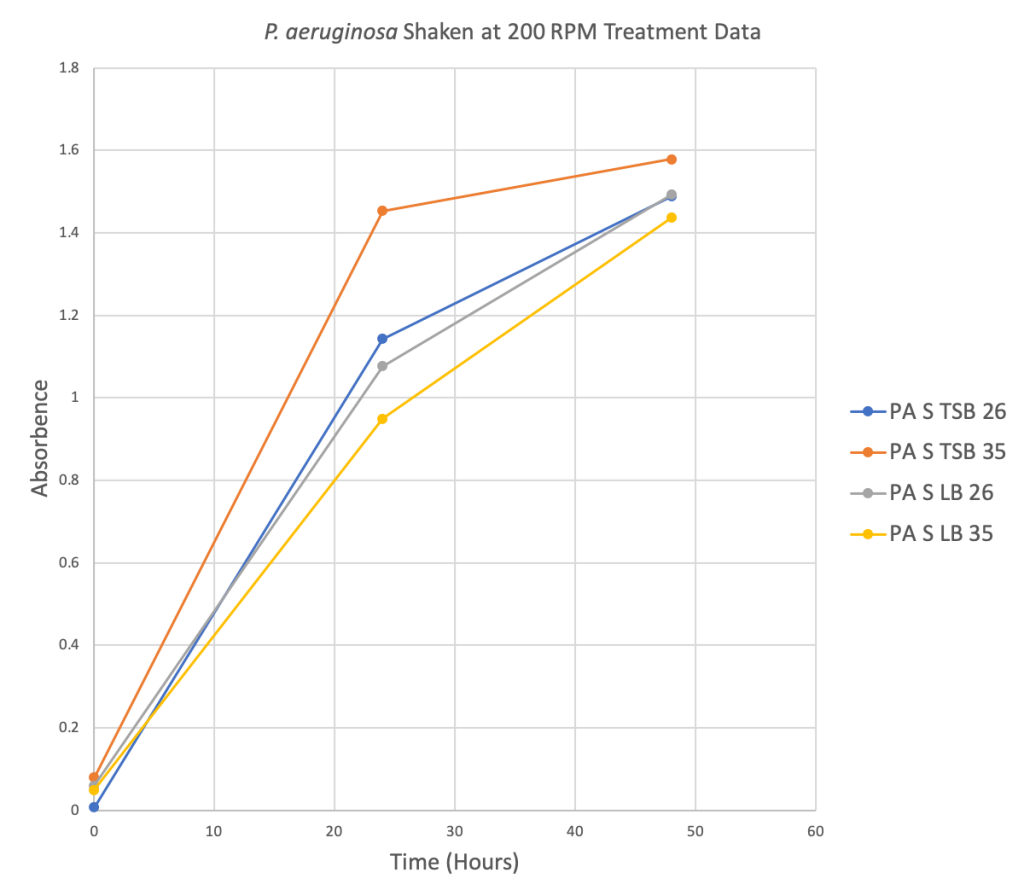
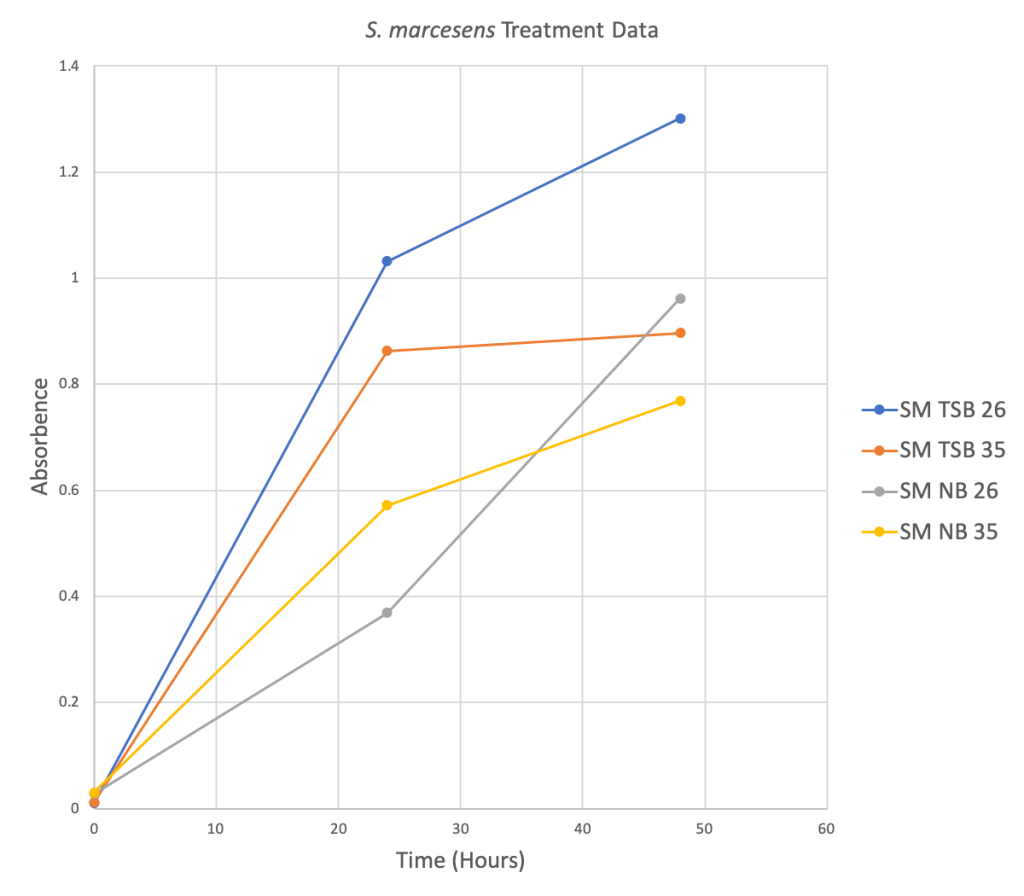
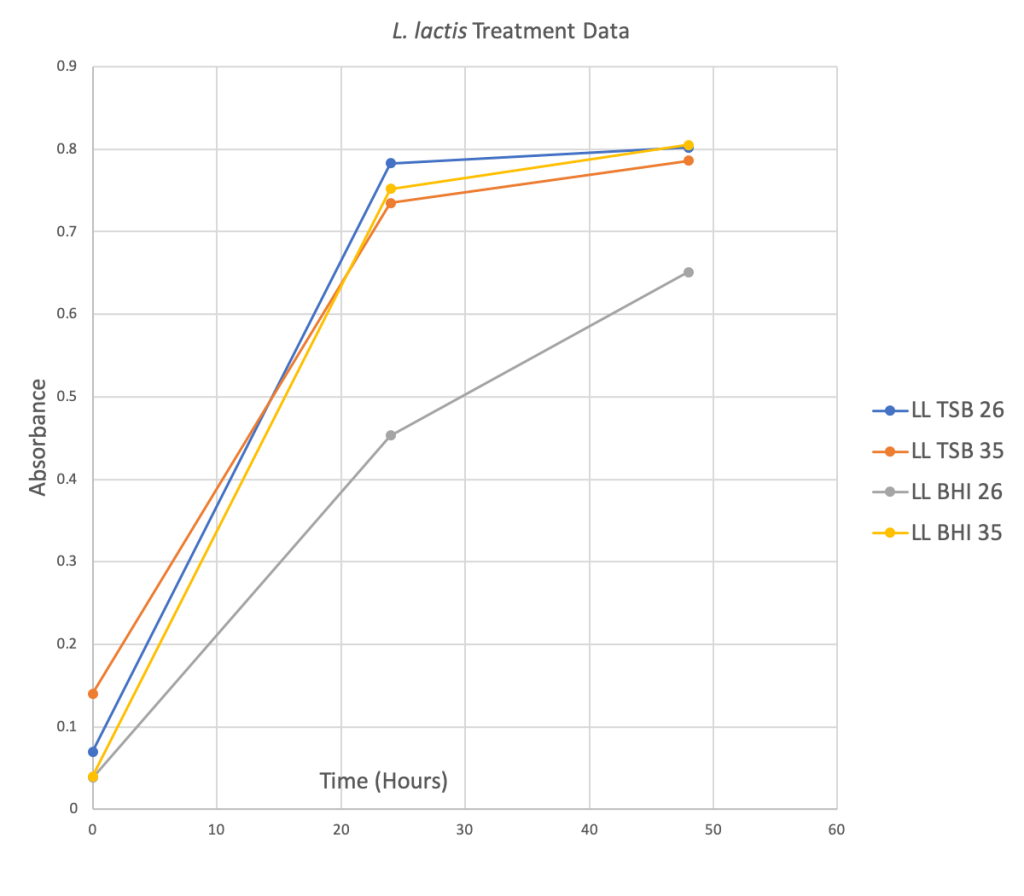
Phase Two: TTC Preliminary Data
For the four weeks the the experiment was run, all samples exhibited the red pigment from the TTC 0.05% solution.
Discussion and Future Direction
Phase One
- Correlation between type of growth media, temperature and the amount of growth.
- L. lactis and S. marcescens both did better in the traditional growth media TSB at 26 degrees Celsius.
- P. aeruginosa not shaken at 200 RPM, did best in LB at 35 degrees
- P. aeruginosa shaken at 200 RPM did best in TSB at 35 degrees Celsius.
Unable to complete Phase Two
- Found that TTC 0.05% that produces a red pigment where there are actively dividing cells – could potentially be used to indicate if a plate is still viable.
In the future we hope to continue this research by considering several more growth conditions. We would like to see the effect of storing the fastidious organisms in broth instead of storing them on plates. We would also like to add a treatment to consider whether the bacteria is best grown anaerobically and aerobically. Lastly, we would like to expand the types of media we would try to store these organisms on.
Additional Information
For questions or comments about this research, access abstract and references or contact Savannah Temple at savannahtemple@u.boisestate.edu.