Brandi Sweet, Miranda Fischer, Erica Rowe, Dr. Stephanie Hall, Dr. Luke Montrose

Introduction
Alzheimer’s Disease (AD) is a progressive neurological disorder that was discovered in 1906 by Dr. Alios Alzheimer who identified the disease physiology as the presence of amyloid plaques, tau proteins, and extensive neuronal loss. Although genetic determinants of AD have been identified, it accounts for only about 5% of the 33.9 million cases worldwide. Compelling studies like Plassman et al., which looked at AD incidence among twins reveal that environmental factors such as diet and exercise may play an important role in disease development.
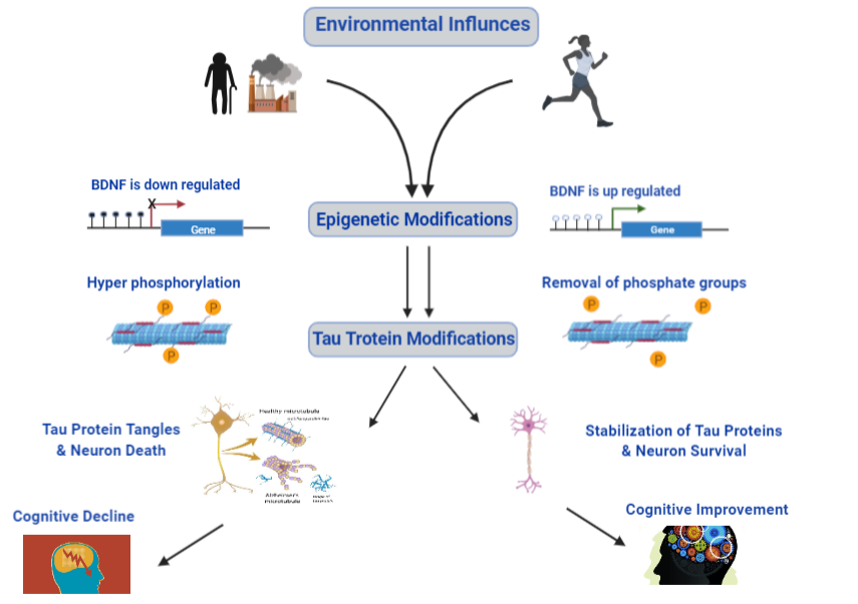
Epigenetic modifications regulate gene expression without altering genetic sequences and can be influenced by environment. To further understand how exercise may influence epigenetics in the context of AD, I conducted a literature review. I found that the BDNF/TrkB pathway is highly regulated by both exercise and epigenetic alterations (e.g., histone modification and DNA methylation). Further, multiple studies noted a common change in percent methylation of the promoter regions of Exon IV of BDNF with exercise. The identification of epigenetically labile regions is important, as it sets the stage for future medical treatments and/or behavioral intervention. Thus, we designed an experimental model to further evaluate the molecular mechanisms unpinning the neuroprotective effects of exercise in AD.
Epigenetic Modifications
1. Histone Modifications
The addition of an acetyl group to the histone tail relaxes the chromatin allowing transcription to occur.
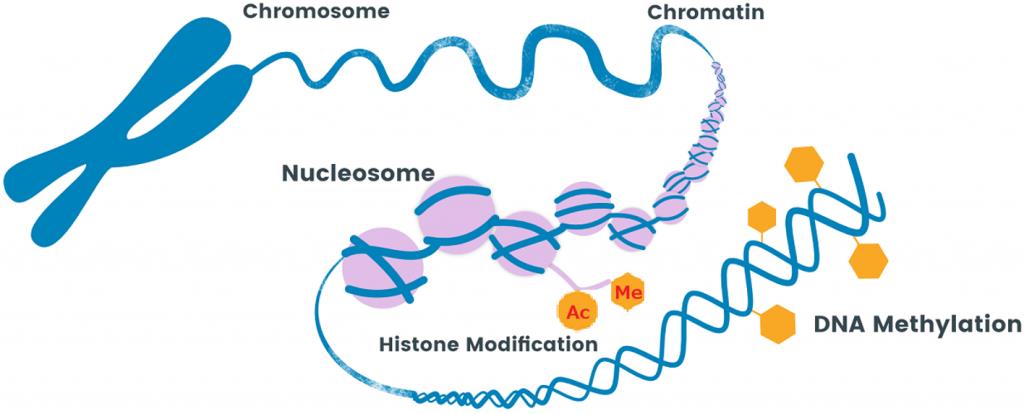
2. DNA Methylation
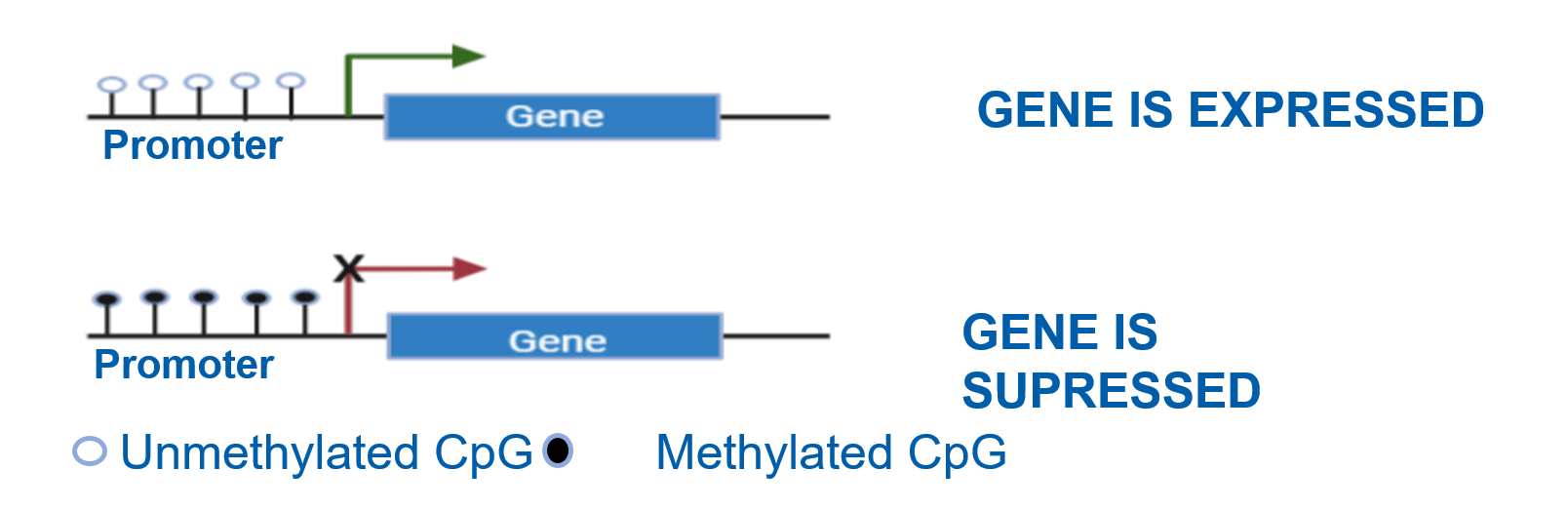
A methyl group attaches to a cytosine (C) that is bound to an adjacent Guanine (G) through a phosphodiester bond, this is known as a CpG site. Methylation down regulated gene expression by blocking transcription binding sites.
Working Disease Model
Proposed Research Methods
TgF344-AD rat model is the optimal research model for AD because these rats displays all three AD pathology markers– amyloid B plaques, tau proteins and extensive neuronal loss.
Treatment Groups:
- Wild Type Treadmill Trained (n = 8-10)
- Wild Type Sedentary ( n= 8-10)
- TgF344-AD Treadmill Trained (n = 8-10)
- TgF344-AD Sedentary (n = 8-10)
Exercise Regimen:
- Begin 6-month treadmill training at 12 months of age
- Treadmill training will be administered 5 days a week
Methods
A literature review was conducted via PubMed and Science Direct with search terms Alzheimer’s Disease, BDNF, epigenetic modifications and exercise. We limited our search to relevant primary literature articles published in the last 3 years. After reviewing the abstracts of 1,251 articles were identified using the search parameters, we selected 21 articles for this review.
Supporting Data
Exercise induced demethylation of BDNF exon iv in a rat model
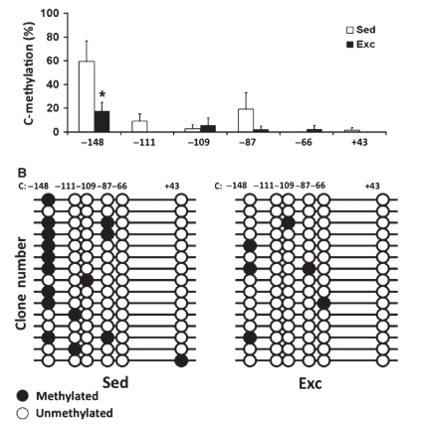
Figure 1. Identifies the greatest % change in methylation 148 base pairs before Exon
Figure 2. Visualization of methylation changes in Sedentary vs Exercised rats.
Discussion
- BDNF is known to be vital for neuroplasticity and cognitive function.
- The BDNF/TrkB pathway appears to play an important role in Tau protein phosphorylation regulation as well as neuron survival.
- Research suggests BDNF/TrkB pathway highly is influenced by exercise.
- Previous work has shown the promoter region of Exon IV of the BDNF gene is susceptible to environmental influence.
Future Research
- More research is needed to elucidate the complete relationship between AD, BDNF, exercise and epigenetics.
- Investigate functional epigenetic modifications that regulate the BDNF/TrkB pathway in an exercise model.
- Identify other genes that may be influenced by exercise, epigenetically modified, and have neuroprotective effects.

References
If you are unable the scan the QR code, but would like access to the references please email Brandi Sweet at: Brandisweet@u.boisestate.edu
Additional Information
For questions or comments about this research, contact Brandi Sweet at Brandisweet@u.boisestate.edu.